Science (English Medium)
Academic Year: 2012-2013
Date: March 2013
Advertisements
How many atoms constitute one unit cell of a face-centered cubic crystal?
Chapter: [0.01] Solid State
Name the method used for the refining of Nickel metal.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What is the covalency of nitrogen in N2O5?
Chapter: [0.01] Solid State
Write the IUPAC name of 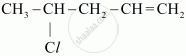
Chapter: [0.05] Coordination Compounds
What happens when \[\ce{CH3 - Br}\] is treated with KCN?
Chapter: [0.06] Haloalkanes and Haloarenes
Write the structure of 3-methyl butanal
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Arrange the following in increasing order of their basic strength in aqueous solution:
\[\ce{CH3NH2, (CH3)3N, (CH3)2NH}\]
Chapter: [0.09] Amines
What are three types of RNA molecules which perform different functions?
Chapter: [0.1] Biomolecules
8 g of glucose, C6H12O6 (Molar Mass = 180 g mol−1) is dissolved in 1 kg of water in a sauce pan. At what temperature will this solution boil?
(Kb for water = 0.52 K kg mol−1, boiling point of pure water = 373.15 K)
Chapter: [0.01] Solutions
The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm−1. Calculate its molar conductivity.
Chapter: [0.02] Electrochemistry
Write the dispersed phase and dispersion medium of the following colloidal systems:
(i) Smoke
(ii) Milk
Chapter: [0.05] Surface Chemistry
What are lyophilic and lyophobic colloids ? Which of these sols can be easily coagulated on the addition of small amounts of electrolytes?
Chapter: [0.05] Surface Chemistry
Write the differences between physisorption and chemisorption with respect to the following:
(i) Specificity
(ii) Temperature dependence
(iii) Reversibility and
(iv) Enthalpy change
Chapter: [0.05] Surface Chemistry
Which solution is used for the leaching of silver metal in the presence of air in the metallurgy of silver?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Out of C and CO, which is a better reducing agent at the lower temperature range in the blast furnace to extract iron from the oxide ore?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What happens when
(i) PCl5 is heated ? Write the reactions involved.
Chapter: [0.07] P - Block Elements
What happens when
H3PO3 is heated ?
Write the reactions involved.
Chapter: [0.07] P - Block Elements
Which metal in the first transition series (3d series) exhibits + 1 oxidation state most frequently and why?
Chapter: [0.04] d-block and f-block Elements
Which of the following cations are coloured in aqueous solutions and why ?
Sc3+, V3+, Ti4+, Mn2+ (At. Nos. Sc = 21, V = 23, Ti = 22, Mn = 25)
Chapter: [0.04] d-block and f-block Elements
Advertisements
Chlorobenzene is extremely less reactive towards a nucleophilic substitution reaction. Give two reasons for the same.
Chapter: [0.06] Haloalkanes and Haloarenes
Explain the mechanism of the following reaction:
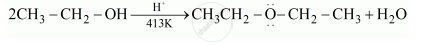
Chapter: [0.07] Alcohols, Phenols and Ethers
How will you convert: Propene to Propan-2-ol?
Chapter: [0.07] Alcohols, Phenols and Ethers
How will you convert: Phenol to 2, 4, 6 − trinitrophenol?
Chapter: [0.07] Alcohols, Phenols and Ethers
What type of semiconductor is obtained when silicon is doped with boron?
Chapter: [0.01] Solid State
What type of magnetism is shown in the following alignment of magnetic moments?

Chapter: [0.01] Solid State
What type of point defect is produced when AgCl is doped with CdCl2?
Chapter: [0.01] Solid State
Determine the osmotic pressure of a solution prepared by dissolving 2.5 × 10−2 g of K2SO4 in 2L of water at 25°C, assuming that it is completely dissociated.
(R = 0.0821 L atm K−1 mol−1, Molar mass of K2SO4 = 174 g mol−1)
Chapter: [0.01] Solutions
Calculate the e.m.f. of the following cell at 298 K:
Fe(s) | Fe2+ (0.001 M) | | H+ (0.01 M) | H2(g) (1 bar) | Pt(s)
Given that \[\ce{E^0_{cell}}\] = 0.44 V
[log 2 = 0.3010, log 3 = 0.4771, log 10 = 1]
Chapter: [0.02] Electrochemistry
How would you account for the following?
Transition metals exhibit variable oxidation states.
Chapter: [0.04] d-block and f-block Elements
How would you account for the following?
Zr (Z = 40) and Hf (Z = 72) have almost identical radii.
Chapter: [0.04] d-block and f-block Elements
How would you account for the following?
Transition metals and their compounds act as catalysts.
Chapter: [0.04] d-block and f-block Elements
Complete the following chemical equations:
`(i) Cr_2O_7^(2-)+6Fe^(2+)+14H^+ ->`
`(ii) 2CrO_4^(2-)+2H^+ ->`
`(iii) 2MnO_4^-+5C_2O_4^(2-)+16H^+ ->`
Chapter: [0.04] d-block and f-block Elements
Write the IUPAC names of the following coordination compounds: [Cr(NH3)3Cl3]
Chapter: [0.05] Coordination Compounds
Write the IUPAC name of the following coordination compound:
K3[Fe(CN)6]
Chapter: [0.05] Coordination Compounds
Write the IUPAC names of the following coordination compounds:
[CoBr2(en)2]+, (en = ethylenediamine)
Chapter: [0.05] Coordination Compounds
Give the structures of A, B and C in the following reactions:
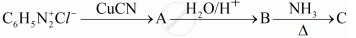
Chapter: [0.09] Amines
Give the structures of A, B and C in the following reaction:
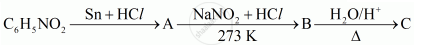
Chapter: [0.09] Amines
Write the names and structure of the monomers of the following polymers: Buna − S
Chapter:
Write the names and structure of the monomers of the following polymers: Neoprene
Chapter:
Write the names and structure of the monomers of the following polymers: Nylon-6, 6
Chapter: [0.15] Polymers
After watching a programme on TV about the adverse effects of junk food and soft drinks on the health of school children, Sonali, a student of Class XII, discussed the issue with the school principal. Principal immediately instructed the canteen contractor to replace the fast food with the fibre and vitamins rich food like sprouts, salad, fruits etc. This decision was welcomed by the parents and the students.
After reading the above passage, answer the following questions:
(a) What value are expressed by Sonali and the Principal of the school?
(b) Give two examples of water-soluble vitamins.
Chapter: [0.1] Biomolecules
Which one of the following is a food preservative?
Equanil, Morphine, Sodium benzoate
Chapter: [0.16] Chemistry in Everyday Life
Why is bithional added to soap?
Chapter: [0.16] Chemistry in Everyday Life
What use of drugs is used in sleeping pills?
Chapter: [0.16] Chemistry in Everyday Life
Advertisements
A reaction is second order in A and first order in B.
(i) Write the differential rate equation.
(ii) How is the rate affected on increasing the concentration of A three times?
(iii) How is the rate affected when the concentrations of both A and B are doubled?
Chapter: [0.03] Chemical Kinetics
A first order reaction takes 40 minutes for 30% decomposition. Calculate t1/2 for this reaction. (Given log 1.428 = 0.1548)
Chapter: [0.03] Chemical Kinetics
For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction.
Chapter: [0.03] Chemical Kinetics
(b) Rate constant ‘k’ of a reaction varies with temperature ‘T’ according to the equation:
`logk=logA-E_a/2.303R(1/T)`
Where Ea is the activation energy. When a graph is plotted for `logk Vs. 1/T` a straight line with a slope of −4250 K is obtained. Calculate ‘Ea’ for the reaction.(R = 8.314 JK−1 mol−1)
Chapter: [0.03] Chemical Kinetics
Give reasons for the following:
(i) Bond enthalpy of F2 is lower than that of Cl2.
(ii) PH3 has lower boiling point than NH3.
Chapter: [0.05] Coordination Compounds
Draw the structures of the following molecules: XeF4
Chapter: [0.07] P - Block Elements
Draw the structures of the following molecules: BrF3
Chapter: [0.07] P - Block Elements
Draw the structures of the following molecules: (HPO3)3
Chapter: [0.07] P - Block Elements
Account for the following:
Helium is used in diving apparatus.
Chapter: [0.07] P - Block Elements
Account for the following: Fluorine does not exhibit positive oxidation state.
Chapter: [0.07] P - Block Elements
Account for the following: Oxygen shows catenation behavior less than sulphur.
Chapter: [0.07] P - Block Elements
Draw the structures of the following molecules: XeF2
Chapter: [0.07] P - Block Elements
Draw the structures of the following molecules: H2S2O8
Chapter: [0.07] P - Block Elements
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Give two reasons.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
How will you bring about the following conversions?
Propanone to propane
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
How will you bring about the following conversion?
Benzoyl chloride to benzaldehyde
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
How will you bring about the following conversion?
Ethanal to but-2-enal
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Complete the following reactions:
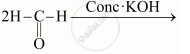
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Complete the following reactions:
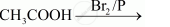
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Complete the following reactions:
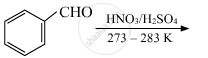
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Give a simple chemical test to distinguish between the following pair of compounds:
Ethanal and Propanal
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Give simple chemical tests to distinguish between the following pairs of compounds: Benzoic acid and Phenol
Chapter: [0.07] Alcohols, Phenols and Ethers
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 12 Chemistry with solutions 2012 - 2013
Previous year Question paper for CBSE Class 12 -2013 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 12.
How CBSE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
