Advertisements
Advertisements
Question
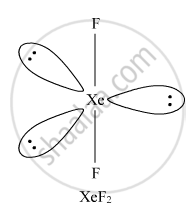
Draw the structures of the following molecules: XeF2
Solution
XeF2, Linear
APPEARS IN
RELATED QUESTIONS
What happens when: XeF4 reacts with SbF5?
Which noble gas has the lowest boiling point?
Write the structures of the following molecules: XeOF4
Fill in the blanks by choosing the appropriate word/words from the brackets
(square pyramidal, electrical, 74; 26, sp3d2, sp3d, chemical, 68, 32, tetrahedral, yellow, white, iodoform, Lucas)
The geometry of XeOF4 molecule is ______ and the hybridisation of Xenon atom in the molecule is ________.
The number of lone pairs of electrons present in ClF5:
Explain the trend in the following atomic properties of group 16 elements:
Electron gain enthalpy
Partial hydrolysis of XeF4 gives ____________.
Helium is preferred to be used in balloons instead of hydrogen because it is ____________.
Substance having the lowest boiling point ______.
Write two uses of neon.
