Science (English Medium)
Academic Year: 2014-2015
Date: March 2015
Advertisements
What is the formula of a compound in which the element Y forms hcp lattice and atoms of X occupy 2/3rd of tetrahedral voids?
Chapter: [0.01] Solid State
Write the IUPAC name of the given compound :
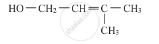
Chapter: [0.09] Amines
Physisorption is reversible while chemisorption is irreversible. Why ?
Chapter: [0.05] Surface Chemistry
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
Chapter: [0.06] Haloalkanes and Haloarenes
Which allotrope of sulphur is thermally stable at room temperature ?
Chapter: [0.07] P - Block Elements
Following reactions occur at cathode during the electrolysis of aqueous copper(II) chloride solution :
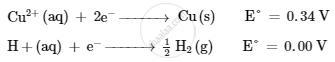
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why ?
Chapter: [0.02] Electrochemistry
State Kohlrausch law of independent migration of ions.
Chapter: [0.02] Electrochemistry
Why do transition elements show variable oxidation states ? In 3d series (Sc to Zn), which elements shows the maximum number of oxidation state and why ?
Chapter: [0.04] d-block and f-block Elements
Write down the IUPAC name of the following complex :
[Cr(en)3]Cl3
Chapter: [0.05] Coordination Compounds
Using IUPAC norms, write the formula for the following:
Potassium tetrachloridopalladate(II)
Chapter: [0.05] Coordination Compounds
Name the reagents used in the following reactions:

Chapter: [0.07] Alcohols, Phenols and Ethers
Name the reagents used in the following reactions:
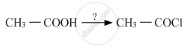
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
What is meant by negative deviation from Raoult's law? Give an example. What is the sign of ∆mixH for negative deviation?
Chapter: [0.01] Solutions
What type of azeotrope is formed by positive deviation from Raoult's law ? Give an example.
Chapter: [0.01] Solutions
Why are alkyl halides insoluble in water?
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Why is Butan-1-ol optically inactive but Butan-2-ol is optically active?
Chapter: [0.05] Coordination Compounds
Although chlorine is an electron withdrawing group, yet it is ortho-, para- directing in electrophilic aromatic substitution reactions. Why?
Chapter: [0.02] Electrochemistry
How will you bring about the following conversion in not more than two steps?
Benzoic acid to Benzaldehyde
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
How do you convert the following :
Ethyne to Ethanal
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
How do you convert the following :
Acetic acid to Methane
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the equations involved in the following reactions :
Stephen reaction
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the equations involved in the following reactions :
Wolff-Kishner reduction
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Advertisements
Write the equations involved in the following reactions :
Etard reaction
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Calculate the mass of NaCl (molar mass = 58.5 g mol−1) to be dissolved in 37.2 g of water to lower the freezing point by 2°C, assuming that NaCl undergoes complete dissociation. (Kf for water = 1.86 K kg mol−1)
Chapter: [0.01] Solutions
Write the names and structures of the monomers of the following polymers:
(i) Terylene
(ii) Bakelite
(iii) Buna-S
Chapter: [0.15] Polymers
Which one of the following is a monosaccharide:
starch, maltose, fructose, cellulose
Chapter: [0.1] Biomolecules
What is the difference between acidic amino acids and basic amino acids?
Chapter: [0.1] Biomolecules
Write the name of the vitamin whose deficiency causes bleeding of gums.
Chapter: [0.1] Biomolecules
Draw the geometrical isomers of complex \[\ce{[Pt(en)2Cl2]^2+}\].
Chapter: [0.05] Coordination Compounds
On the basis of crystal field theory, write the electronic configuration for d4 ion if ∆0 < P.
Chapter: [0.05] Coordination Compounds
Write the hybridization type and magnetic behaviour of the complex [Ni(CN)4]2−. (Atomic number of Ni = 28)
Chapter: [0.05] Coordination Compounds
Indicate the principle behind the method used for the refining of Nickel.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What is the role of dilute NaCN in the extraction of gold ?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What is 'copper matte' ?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Calculate the emf of the following cell at 25°C :
Chapter: [0.02] Electrochemistry
Predict the products of the following reactions :
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Predict the products of the following reactions :
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
An element X (molar mass = 60 g mol−1) has a density of 6.23 g cm−3. Identify the type of cubic unit cell, if the edge length of the unit cell is 4 ✕ 10−8 cm.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
How would you account the following :
Highest fluoride of Mn is MnF4 whereas the highest oxide is Mn2O7.
Chapter: [0.04] d-block and f-block Elements
How would you account the following :
Transition metals and their compounds show catalytic properties.
Chapter: [0.06] Haloalkanes and Haloarenes
Complete the following equation :
Chapter: [0.04] d-block and f-block Elements
Give reasons for the following observations :
A delta is formed at the meeting point of sea water and river water.
Chapter: [0.02] Electrochemistry
Give reasons for the following observations :
NH3 gas adsorbs more readily than N2 gas on the surface of charcoal.
Chapter: [0.05] Surface Chemistry
Give reasons for the following observations :
Powdered substances are more effective adsorbents.
Chapter: [0.05] Surface Chemistry
Seeing the growing cases of diabetes and depression among children, Mr. Chopra, the principal of one reputed school organized a seminar in which he invited parents and principals. They all resolved this issue by strictly banning the junk food in schools and by introducing healthy snacks and drinks like soup, lassi, milk etc. in school canteens. They also decided to make compulsory half an hour physical activities for the students in the morning assembly daily. After six months, Mr. Chopra conducted the health survey in most of the schools and discovered a tremendous improvement in the health of students.
After reading the above passage, answer the following questions :
(i) What are the values (at least two) displayed by Mr. Chopra ?
(ii) As a student, how can you spread awareness about this issue ?
(iii) Why should antidepressant drugs not be taken without consulting a doctor ?
(iv) Give two examples of artificial sweeteners.
Chapter: [0.16] Chemistry in Everyday Life
Advertisements
Illustrate the following reactions giving suitable example in each case
Ammonolysis
Chapter: [0.09] Amines
Illustrate the following reactions giving suitable example in each case
Coupling reaction
Chapter: [0.09] Amines
Illustrate the following reactions giving suitable example in each case
Acetylation of amines
Chapter: [0.09] Amines
Describe Hinsberg method for the identification of primary, secondary and tertiary amines. Also write the chemical equations of the reactions involved.
Chapter: [0.09] Amines
Write the structures of main products when benzene diazonium chloride (C6 H5 N+2CI−)C6 H5 N2+CI- reacts with the : HBF4/∆
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the structures of main products when benzene diazonium chloride (C6 H5 N+2CI−)C6 H5 N2+CI- reacts with the :Cu/HBr
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the structures of A, B and C in the following reactions :
\[ C_6 H_5 {NO}_2 \to^{Sn/HCI} A \to^{{NaNO}_2 /HCI}_{273 K} B \to^{H_2 O}_∆ C\]
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the structures of A, B and C in the following reactions :
Chapter: [0.07] Alcohols, Phenols and Ethers
Define activation energy.
Chapter: [0.03] Chemical Kinetics
Define the following terms: Rate constant
Chapter: [0.03] Chemical Kinetics
A first order reaction takes 10 minutes for 25% decomposition. Calculate t1/2 for the reaction.
(Given : log 2 = 0.3010, log 3 = 0.4771, log 4 = 0.6021)
Chapter: [0.03] Chemical Kinetics
For a chemical reaction R → P, the variation in the concentration (R) vs. time (t) plot is given as:
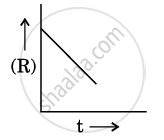
(i) Predict the order of the reaction.
(ii) What is the slope of the curve ?
(iii) Write the unit of rate constant for this reaction.
Chapter: [0.03] Chemical Kinetics
Show that the time required for 99% completion is double of the time required for the completion of 90% reaction.
Chapter: [0.03] Chemical Kinetics
Account for the following :
Bond angle in NH+4NH4+ is higher than NH3.
Chapter: [0.05] Coordination Compounds
Account for the following :
H2S has lower boiling point than H2O.
Chapter: [0.01] Solutions
Account for the following :
Reducing character decreases from SO2 to TeO2.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Draw the structures of the following: H4P2O7 (Pyrophosphoric acid)
Chapter: [0.07] P - Block Elements
Draw the structures of the following molecules: XeF2
Chapter: [0.07] P - Block Elements
Draw the structures of the following molecules: XeF4
Chapter: [0.07] P - Block Elements
Draw the structures of the following :
H2S2O7
Chapter: [0.07] P - Block Elements
Account for the following :
Iron on reaction with HCl forms FeCl2 nd not FeCl3.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Account for the following :
HClO4 is a stronger acid than HClO.
Chapter: [0.07] P - Block Elements
Account for the following :
BiH3 is the strongest reducing agent amongst all the hydrides of group 15.
Chapter: [0.07] P - Block Elements
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 12 Chemistry with solutions 2014 - 2015
Previous year Question paper for CBSE Class 12 -2015 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 12.
How CBSE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
