Advertisements
Advertisements
Question
Following reactions occur at cathode during the electrolysis of aqueous copper(II) chloride solution :
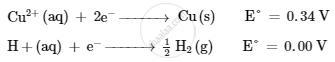
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why ?
Solution
\[{Cu}^{2 +} \left( aq \right) + 2 e^- \to Cu\left( s \right) E^{\circ} = + 0 . 34 V\]
\[ H^+ \left( aq \right) + e^- \to \frac{1}{2} H_2 \left( g \right) E^{\circ} = 0 . 00 V\]
The relationship between the standard free energy change and the standard emf of a cell reaction is given by
Thus, the more positive the standard reduction potential of a reaction, the more negative is the standard free energy change associated with the process and, consequently, the higher is the feasibility of the reaction.
Since E∘Cu2+/CuECu2+/Cu° has a greater positive value than E∘H+/HEH+/H°, the reaction that is feasible at the cathode is
Cu2+(aq) + 2e− → Cu(s)
APPEARS IN
RELATED QUESTIONS
Number of faradays of electricity required to liberate 12 g of hydrogen is:
Using the E° values of A and B, predict which is better for coating the surface of iron [E°(Fe+2/Fe) = -0.44V] to prevent corrosion and why?
Given: E° (A+2/A)=-2.37 V: E°(B+2/B)= -0.14V
Following reactions occur at cathode during the electrolysis of aqueous sodium chloride solution:
Na+(aq) + e− ⟶ Na (s) E0 = 2.71 V
H+(aq) + e− ⟶ `1/2` H2 (g) E0 = 0.00 V
On the basis of their standard reduction electrode potential (E0) values, which reaction is feasible at the cathode and why?
How much charge is required for the following reduction:
1 mol of \[\ce{MnO^-_4}\] to \[\ce{Mn^{2+}}\]?
On passing 1.5 F charge, the number of moles of aluminium deposited at cathode are _______ [Molar mass of Al = 27 gram mol–1]
(A) 1.0
(B) 13.5
(C) 0.50
(D) 0.75
Draw neat labelled diagram of electrolytic refining of blister copper
What is the ratio of volumes of H2 and O2 liberated during electrolysis of acidified water?
(A) 1 : 2
(B) 2 : 1
(C) 1 : 8
(D) 8 : 1
In the electrolysis of aqueous sodium chloride solution which of the half cell reaction will occur at anode?
What will happen during the electrolysis of aqueous solution of \[\ce{CuSO4}\] by using platinum electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will deposit at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will dissolve at anode.
On Electrolysis of dilute sulphuric acid using platinum electrodes, the product obtained at the anode will be.
