Advertisements
Advertisements
Question
In the electrolysis of aqueous sodium chloride solution which of the half cell reaction will occur at anode?
Options
\[\ce{Na+ (aq) + e- -> Na (s); E^{Θ}_{cell} = - 2.71V}\]
\[\ce{2H2O (l) -> O2(g) + 4H+ (aq) + 4e^- ; E^{Θ}_{cell} = 1.23V}\]
\[\ce{H^+ (aq) + e^- -> 1/2 H2 (g); E^{Θ}_{cell} = 0.00V}\]
\[\ce{Cl^- (aq) -> 1/2 Cl2 (g) + e^- ; E^{Θ}_{cell} = 1.36V}\]
Solution
\[\ce{Cl^- (aq) -> 1/2 Cl2 (g) + e^- ; E^{Θ}_{cell} = 1.36V}\]
Explanation:
During electrolysis of aqueous
\[\ce{NaCl -> Na^+ + Cl^-}\]
\[\ce{H2O -> H+ + OH-}\]
\[\ce{Na+ + e- -> Na (E^{Θ}_{cell} = - 2.71V)}\]
\[\ce{H^+ e- -> 1/2 H2 E^{Θ}_{cell} = 0.00V}\]
At cathode,
\[\ce{H2O + e- -> 1/2 H2 + OH-}\]
At anode, two reactions are possible.
\[\ce{Cl^{-} -> 1/2 Cl2 + e- ; E^{Θ}_{cell} = 1.36V}\]
\[\ce{2H2O -> O2 + 4H+ + 4e- ; E^{Θ}_{cell} = 1.23V}\]
APPEARS IN
RELATED QUESTIONS
The charge of how many coulomb is required to deposit 1.0 g of sodium metal (molar mass 23.0 g mol-1) from sodium ions is -
- 2098
- 96500
- 193000
- 4196
How much charge is required for the following reduction:
1 mol of \[\ce{MnO^-_4}\] to \[\ce{Mn^{2+}}\]?
What is the ratio of volumes of H2 and O2 liberated during electrolysis of acidified water?
(A) 1 : 2
(B) 2 : 1
(C) 1 : 8
(D) 8 : 1
State second law of electrolysis
Solve the following question.
A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4 and ZnSO4 until 2.8 g of Fe deposited at the cathode of cell X. How long did the current flow? Calculate the mass of Zn deposited at the cathode of cell Y.
(Molar mass : Fe = 56 g mol–1, Zn = 65.3 g mol–1, 1F = 96500 C mol–1)
Electrolytic cell uses electrical energy to bring about ____________.
Aqueous copper sulphate solution and aqueous silver nitrate solution are electrolysed by 1 ampere current for 10 minutes in separate electrolytic cells. Will the mass of copper and silver deposited on the cathode be same or different? Explain your answer.
Consider the figure and answer the following question.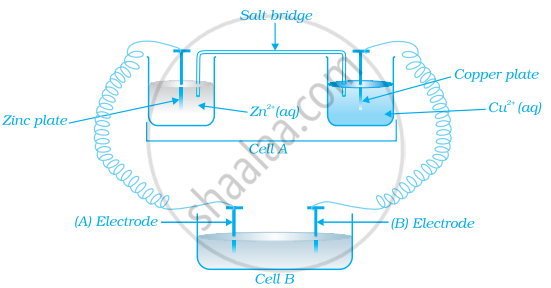
Cell ‘A’ has ECell = 2V and Cell ‘B’ has ECell = 1.1V which of the two cells ‘A’ or ‘B’ will act as an electrolytic cell. Which electrode reactions will occur in this cell?
Given `1/a` = 0.5 CM–1, R = 50 ohm, N = 1.0 then equivalent conductance of electrolytic cell is
A current of 4 amp was passed for 2 hours through a solution of copper sulphate when 5.0 g of copper was deposited. The current efficiency is ______% (Cu = 63.5).
