Advertisements
Advertisements
Question
The positive value of the standard electrode potential of Cu2+/Cu indicates that:
(i) this redox couple is a stronger reducing agent than the H+/H2 couple.
(ii) this redox couple is a stronger oxidising agent than H+/H2 .
(iii) Cu can displace H2 from acid.
(iv) Cu cannot displace H2 from acid.
Solution
(ii) this redox couple is a stronger oxidising agent than H+/H2 .
(iv) Cu cannot displace H2 from acid.
Explanation:
The lesser the E0 value of the redox couple higher is the reducing power.
For \[\ce{Cu^{2+} + 2e^{-} -> Cu ; E^0 = 0.34 V}\]
For \[\ce{2H^{+} + 2e^{-} -> H ; E^0 = 0.00 V}\]
Since the second redox couple has less standard reduction potential than the first so it can be concluded that the redox couple is a stronger oxidizing agent than H+/H2 and copper cannot H2 from acid.
APPEARS IN
RELATED QUESTIONS
Calculate Ecell and ΔG for the following at 28°C :
Mg(s) + Sn2+( 0.04M ) → Mg2+( 0.06M ) + Sn(s)
E°cell = 2.23V. Is the reaction spontaneous ?
Can copper sulphate solution be stored in an iron vessel? Explain.
Calculate emf of the following cell at 25°C:
\[\ce{Sn/Sn^2+ (0.001 M) || H+ (0.01 M) | H2_{(g)} (1 bar) | Pt_{(s)}}\]
Given: \[\ce{E^\circ(Sn^2+/sn) = -0.14 V, E^\circ H+/H2 = 0.00 V (log 10 = 1)}\]
Given the standard electrode potentials,
\[\ce{K+/K}\] = −2.93 V, \[\ce{Ag+/Ag}\] = 0.80 V,
\[\ce{Hg^{2+}/Hg}\] = 0.79 V
\[\ce{Mg^{2+}/Mg}\] = −2.37 V, \[\ce{Cr^{3+}/Cr}\] = −0.74 V
Arrange these metals in their increasing order of reducing power.
Depict the galvanic cell in which the reaction \[\ce{Zn(s) + 2Ag+(aq) → Zn^{2+}(aq) + 2Ag(s)}\] takes place. Further show:
- Which of the electrode is negatively charged?
- The carriers of the current in the cell.
- Individual reaction at each electrode.
Using the data given below find out the strongest reducing agent.
`"E"_("Cr"_2"O"_7^(2-)//"Cr"^(3+))^⊖` = 1.33 V `"E"_("Cl"_2//"Cl"^-) = 1.36` V
`"E"_("MnO"_4^-//"Mn"^(2+))` = 1.51 V `"E"_("Cr"^(3+)//"Cr")` = - 0.74 V
What does the negative sign in the expression `"E"^Θ ("Zn"^(2+))//("Zn")` = − 0.76 V mean?
Value of standard electrode potential for the oxidation of \[\ce{Cl-}\] ions is more positive than that of water, even then in the electrolysis of aqueous sodium chloride, why is \[\ce{Cl-}\] oxidised at anode instead of water?
Consider the figure and answer the following question.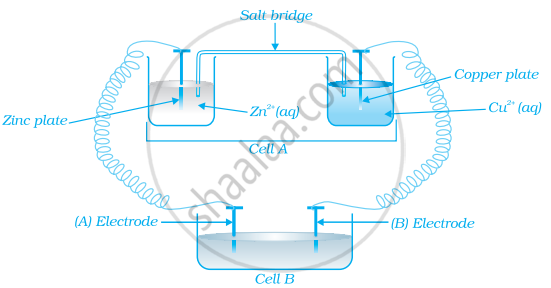
If cell ‘A’ has ECell = 0.5V and cell ‘B’ has ECell = 1.1V then what will be the reactions at anode and cathode?
Represent the cell in which the following reaction takes place.The value of E˚ for the cell is 1.260 V. What is the value of Ecell?
\[\ce{2Al (s) + 3Cd^{2+} (0.1M) -> 3Cd (s) + 2Al^{3+} (0.01M)}\]
