Advertisements
Advertisements
Question
Depict the galvanic cell in which the reaction \[\ce{Zn(s) + 2Ag+(aq) → Zn^{2+}(aq) + 2Ag(s)}\] takes place. Further show:
- Which of the electrode is negatively charged?
- The carriers of the current in the cell.
- Individual reaction at each electrode.
Solution
The set-up will be similar to as shown below:
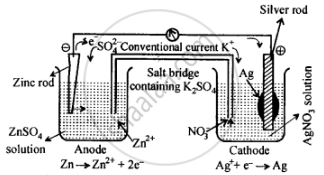
The cell will be represented as:
\[\ce{Zn(s) | Zn^{2+}(aq) || Ag^+(aq) | Ag(s)}\]
- Anode, i.e., zinc electrode, will be negatively charged.
- The current will flow from silver to zinc in the external circuit.
- At anode: \[\ce{Zn(s) -> Zn^{2+}(aq) + 2e–}\]
At cathode: \[\ce{Ag+(aq) + e– -> Ag(s)}\]
APPEARS IN
RELATED QUESTIONS
Draw a neat and well labelled diagram of primary reference electrode.
Arrange the following reducing agents in the order of increasing strength under standard state conditions. Justify the answer
|
Element |
Al(s) |
Cu(s) |
Cl(aq) |
Ni(s) |
|
Eo |
-1.66V |
0.34V |
1.36V |
-0.26V |
Calculate E°cell for the following reaction at 298 K:
2Al(s) + 3Cu+2(0.01M) → 2Al+3(0.01M) + 3Cu(s)
Given: Ecell = 1.98V
Calculate e.m.f of the following cell at 298 K:
2Cr(s) + 3Fe2+ (0.1M) → 2Cr3+ (0.01M) + 3 Fe(s)
Given: E°(Cr3+ | Cr) = – 0.74 VE° (Fe2+ | Fe) = – 0.44 V
Calculate emf of the following cell at 25 °C :
Fe|Fe2+(0.001 M)| |H+(0.01 M)|H2(g) (1 bar)|Pt (s)
E°(Fe2+| Fe)= −0.44 V E°(H+ | H2) = 0.00 V
Calculate e.m.f. and ∆G for the following cell:
Mg (s) |Mg2+ (0.001M) || Cu2+ (0.0001M) | Cu (s)
`"Given :" E_((Mg^(2+)"/"Mg))^0=−2.37 V, E_((Cu^(2+)"/"Cu))^0=+0.34 V.`
Given the standard electrode potentials,
\[\ce{K+/K}\] = −2.93 V, \[\ce{Ag+/Ag}\] = 0.80 V,
\[\ce{Hg^{2+}/Hg}\] = 0.79 V
\[\ce{Mg^{2+}/Mg}\] = −2.37 V, \[\ce{Cr^{3+}/Cr}\] = −0.74 V
Arrange these metals in their increasing order of reducing power.
In the representation of the galvanic cell, the ions in the same phase are separated by a _______.
Galvanic or a voltaic cell converts the chemical energy liberated during a redox reaction to ____________.
Standard hydrogen electrode operated under standard conditions of 1 atm H2 pressure, 298 K, and pH = 0 has a cell potential of ____________.
Using the data given below find out the strongest reducing agent.
`"E"_("Cr"_2"O"_7^(2-)//"Cr"^(3+))^⊖` = 1.33 V `"E"_("Cl"_2//"Cl"^-) = 1.36` V
`"E"_("MnO"_4^-//"Mn"^(2+))` = 1.51 V `"E"_("Cr"^(3+)//"Cr")` = - 0.74 V
Use the data given in below find out which of the following is the strongest oxidising agent.
`"E"_("Cr"_2"O"_7^(2-)//"Cr"^(3+))^⊖`= 1.33 V `"E"_("Cl"_2//"Cl"^-)^⊖` = 1.36 V
`"E"_("MnO"_4^-//"Mn"^(2+))^⊖` = 1.51 V `"E"_("Cr"^(3+)//"Cr")^⊖` = - 0.74 V
What does the negative sign in the expression `"E"^Θ ("Zn"^(2+))//("Zn")` = − 0.76 V mean?
Assertion: Cu is less reactive than hydrogen.
Reason: `E_((Cu^(2+))/(Cu))^Θ` is negative.
Represent the cell in which the following reaction takes place.The value of E˚ for the cell is 1.260 V. What is the value of Ecell?
\[\ce{2Al (s) + 3Cd^{2+} (0.1M) -> 3Cd (s) + 2Al^{3+} (0.01M)}\]
Standard electrode potential of three metals X, Y and Z are – 1.2 V, + 0.5 V and – 3.0 V, respectively. The reducing power of these metals will be:
The potential of a hydrogen electrode at PH = 10 is
