Advertisements
Advertisements
Question
What does the negative sign in the expression `"E"^Θ ("Zn"^(2+))//("Zn")` = − 0.76 V mean?
Solution
`"E"^Θ ("Zn"^(2+))//("Zn")` = − 0.76 V
Negative sign shows that the reducing power of zinc is greater than that of hydrogen.
\[\ce{Zn + H2SO4 -> ZnSO4 + H2}\]
APPEARS IN
RELATED QUESTIONS
Draw a neat and well labelled diagram of primary reference electrode.
Calculate emf of the following cell at 25°C:
\[\ce{Sn/Sn^2+ (0.001 M) || H+ (0.01 M) | H2_{(g)} (1 bar) | Pt_{(s)}}\]
Given: \[\ce{E^\circ(Sn^2+/sn) = -0.14 V, E^\circ H+/H2 = 0.00 V (log 10 = 1)}\]
Depict the galvanic cell in which the reaction \[\ce{Zn(s) + 2Ag+(aq) → Zn^{2+}(aq) + 2Ag(s)}\] takes place. Further show:
- Which of the electrode is negatively charged?
- The carriers of the current in the cell.
- Individual reaction at each electrode.
Standard hydrogen electrode operated under standard conditions of 1 atm H2 pressure, 298 K, and pH = 0 has a cell potential of ____________.
The positive value of the standard electrode potential of Cu2+/Cu indicates that:
(i) this redox couple is a stronger reducing agent than the H+/H2 couple.
(ii) this redox couple is a stronger oxidising agent than H+/H2 .
(iii) Cu can displace H2 from acid.
(iv) Cu cannot displace H2 from acid.
Assertion: Cu is less reactive than hydrogen.
Reason: `E_((Cu^(2+))/(Cu))^Θ` is negative.
Consider the figure and answer the following question.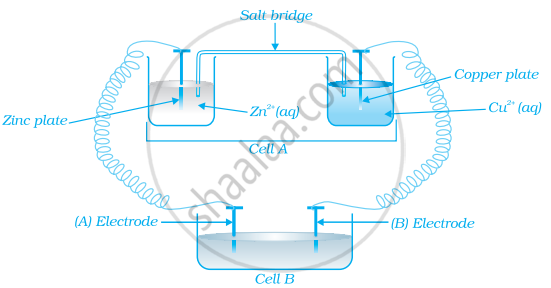
If cell ‘A’ has ECell = 0.5V and cell ‘B’ has ECell = 1.1V then what will be the reactions at anode and cathode?
Represent the cell in which the following reaction takes place.The value of E˚ for the cell is 1.260 V. What is the value of Ecell?
\[\ce{2Al (s) + 3Cd^{2+} (0.1M) -> 3Cd (s) + 2Al^{3+} (0.01M)}\]
The standard electrode potential of the two half cells are given below:
\[\ce{Ni^{2+} + 2e^{-} -> Ni, E_0 = - 0.25 Volt}\]
\[\ce{Zn^{2+} + 2e^{-} -> Zn, E_0 = - 0.77 Volt}\]
The voltage of cell formed by combining the two half cells would be?
Which is the correct order of second ionization potential of C, N, O and F in the following?
