Advertisements
Online Mock Tests
Chapters
2: Solutions
▶ 3: Electrochemistry
4: Chemical Kinetics
5: Surface Chemistry
6: General Principle and Processes of Isolation of Elements
7: The p-block Elements
8: The d-and f-Block Elements
9: Coordination Compounds
10: Haloalkanes and Haloarenes
11: Alcohols, Phenols and Ethers
12: Aldehydes, Ketones and Carboxylic Acids
13: Amines
14: Biomolecules
15: Polymers
16: Chemistry In Everyday Life
![NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 3 - Electrochemistry NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 3 - Electrochemistry - Shaalaa.com](/images/chemistry-english-class-12_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 3: Electrochemistry
Below listed, you can find solutions for Chapter 3 of CBSE NCERT Exemplar for Chemistry [English] Class 12.
NCERT Exemplar solutions for Chemistry [English] Class 12 3 Electrochemistry Exercises [Pages 33 - 43]
Multiple Choice Questions (Type - I)
Which cell will measure standard electrode potential of copper electrode?
\[\ce{Pt(s) | H2 (g,0.1 bar) | H+ (aq; 1 M) || Cu^{2+} (aq;1M) | Cu}\]
\[\ce{Pt(s) | H2 (g, 1 bar) | H+ (aq; 1 M) || Cu^{2+} (aq; 2 M) | Cu}\]
\[\ce{Pt(s) | H2 (g, 1 bar) | H+ (aq; 1 M) || Cu^{2+} (aq; 1 M) | Cu}\]
\[\ce{Pt(s) | H2 (g, 1 bar) | H+ (aq; 0.1 M) || Cu^{2+} (aq; 1 M) | Cu}\]
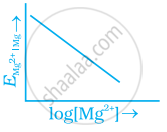
Electrode potential for Mg electrode varies according to the equation
`E_(Mg^(2+) | Mg) = E_(Mg^(2+) | Mg)^Θ - 0.059/2 log 1/([Mg^(2+)])`. The graph of `E_(Mg^(2+) | Mg)` vs `log [Mg^(2+)]` is ______.
Which of the following statement is correct?
ECell and ∆rG of cell reaction both are extensive properties.
ECell and ∆rG of cell reaction both are intensive properties.
ECell is an intensive property while ∆rG of cell reaction is an extensive property.
ECell is an extensive property while ∆rG of cell reaction is an intensive property.
The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called ______.
Cell potential
Cell emf
Potential difference
Cell voltage
Which of the following statement is not correct about an inert electrode in a cell?
It does not participate in the cell reaction.
It provides surface either for oxidation or for reduction reaction.
It provides surface for conduction of electrons.
It provides surface for redox reaction.
An electrochemical cell can behave like an electrolytic cell when ______.
Ecell = 0
Ecell > Eext
Eext > Ecell
Ecell = Eext
Which of the statements about solutions of electrolytes is not correct?
Conductivity of solution depends upon size of ions.
Conductivity depends upon viscosiy of solution.
Conductivity does not depend upon solvation of ions present in solution.
Conductivity of solution increases with temperature.
Using the data given below find out the strongest reducing agent.
`"E"_("Cr"_2"O"_7^(2-)//"Cr"^(3+))^⊖` = 1.33 V `"E"_("Cl"_2//"Cl"^-) = 1.36` V
`"E"_("MnO"_4^-//"Mn"^(2+))` = 1.51 V `"E"_("Cr"^(3+)//"Cr")` = - 0.74 V
Cl–
Cr
Cr3+
Mn2+
Use the data given in below find out which of the following is the strongest oxidising agent.
`"E"_("Cr"_2"O"_7^(2-)//"Cr"^(3+))^⊖`= 1.33 V `"E"_("Cl"_2//"Cl"^-)^⊖` = 1.36 V
`"E"_("MnO"_4^-//"Mn"^(2+))^⊖` = 1.51 V `"E"_("Cr"^(3+)//"Cr")^⊖` = - 0.74 V
Cl–
Mn2+
`"MNO"_4^-`
Cr3+
Use the data given in below find out which option the order of reducing power is correct.
`"E"_("Cr"_2"O"_7^(2-)//"Cr"^(3+))^⊖`= 1.33 V `"E"_("Cl"_2//"Cl"^-)^⊖` = 1.36 V
`"E"_("MnO"_4^-//"Mn"^(2+))^⊖` = 1.51 V `"E"_("Cr"^(3+)//"Cr")^⊖` = - 0.74 V
\[\ce{Cr^{3+} < Cl– < Mn^{2+} < Cr}\]
\[\ce{Mn^{2+} < Cl– < Cr^{3+} < Cr}\]
\[\ce{Cr^{3+} < Cl– < Cr_2O_7^{2–} < MnO^{-}4}\]
\[\ce{Mn2+ < Cr3+ < Cl– < Cr}\]
Use the data given in below find out the most stable ion in its reduced form.
`"E"_("Cr"_2"O"_7^(2-)//"Cr"^(3+))^⊖`= 1.33 V `"E"_("Cl"_2//"Cl"^-)^⊖` = 1.36 V
`"E"_("MnO"_4^-//"Mn"^(2+))^⊖` = 1.51 V `"E"_("Cr"^(3+)//"Cr")^⊖` = - 0.74 V
\[\ce{Cl–}\]
\[\ce{Cr^{3+}}\]
\[\ce{Cr}\]
\[\ce{Mn^{2+}}\]
Use the data given in below find out the most stable oxidised species.
`E^0 (Cr_2O_1^(2-))/(Cr_(3+))` = 1.33 V `E^0 (Cl_2)/(Cl^-)` = 1.36 V
`E^0 (MnO_4^-)/(MN^(2+))` = 1.51 V `E^0 (Cr^(3+))/(Cr)` = – 0.74 V
\[\ce{Cr^{3+}}\]
\[\ce{MnO^{-}4}\]
\[\ce{CrO^{2-}7}\]
\[\ce{Mn^{2+}}\]
The quantity of charge required to obtain one mole of aluminium from Al2O3 is ______.
1F
6F
3F
2F
The cell constant of a conductivity cell ______.
changes with change of electrolyte.
changes with change of concentration of electrolyte.
changes with temperature of electrolyte.
remains constant for a cell.
While charging the lead storage battery ______.
\[\ce{PbSO4}\] anode is reduced to \[\ce{Pb}\].
\[\ce{PbSO4}\] cathode is reduced to \[\ce{Pb}\].
\[\ce{PbSO4}\] cathode is oxidised to \[\ce{Pb}\].
\[\ce{PbSO4}\] anode is oxidised to \[\ce{PbO2}\].
\[\ce{Λ^0_m}_{(NH_4OH)}\] is equal to ______.
\[\ce{Λ^0_m}_{(NH_4OH)} + \ce{Λ^0_m_{(NH_4Cl) } - \ce{Λ^0}_{(HCl)}}\]
\[\ce{Λ^0_m}_{(NH_4Cl)} + \ce{Λ^0_m_{(NaOH) } - \ce{Λ^0}_{(NaCl)}}\]
\[\ce{Λ^0_m}_{(NH_4Cl)} + \ce{Λ^0_m_{(NaCl) } - \ce{Λ^0}_{NaOH)}}\]
\[\ce{Λ^0_m}_{(NaOH)} + \ce{Λ^0_m_{(NACl) } - \ce{Λ^0}_{(NH_4Cl)}}\]
In the electrolysis of aqueous sodium chloride solution which of the half cell reaction will occur at anode?
\[\ce{Na+ (aq) + e- -> Na (s); E^{Θ}_{cell} = - 2.71V}\]
\[\ce{2H2O (l) -> O2(g) + 4H+ (aq) + 4e^- ; E^{Θ}_{cell} = 1.23V}\]
\[\ce{H^+ (aq) + e^- -> 1/2 H2 (g); E^{Θ}_{cell} = 0.00V}\]
\[\ce{Cl^- (aq) -> 1/2 Cl2 (g) + e^- ; E^{Θ}_{cell} = 1.36V}\]
Multiple Choice Questions (Type-II) Note: In the following questions two or more than two options may be correct.
The positive value of the standard electrode potential of Cu2+/Cu indicates that:
(i) this redox couple is a stronger reducing agent than the H+/H2 couple.
(ii) this redox couple is a stronger oxidising agent than H+/H2 .
(iii) Cu can displace H2 from acid.
(iv) Cu cannot displace H2 from acid.
`E_(cell)^Θ` for some half cell reactions are given below. On the basis of these mark the correct answer.
(a) \[\ce{H^{+} (aq) + e^{-} -> 1/2 H_2 (g); E^Θ_{cell} = 0.00V}\]
(b) \[\ce{2H2O (1) -> O2 (g) + 4H^{+} (aq) + 4e^{-}; E^Θ_{cell} = 1.23V}\]
(c) \[\ce{2SO^{2-}_{4} (aq) -> S2O^{2-}_{8} (aq) + 2e^{-}; E^Θ_{cell} = 1.96V}\]
(i) In dilute sulphuric acid solution, hydrogen will be reduced at cathode.
(ii) In concentrated sulphuric acid solution, water will be oxidised at anode.
(iii) In dilute sulphuric acid solution, water will be oxidised at anode.
(iv) In dilute sulphuric acid solution, \[\ce{SO4^{2-}}\] ion will be oxidised to tetrathionate ion at anode.
`E_(cell)^Θ` = 1.1V for Daniel cell. Which of the following expressions are correct description of state of equilibrium in this cell?
(i) 1.1 = `K_c`
(ii) `(2.303RT)/(2F) logK_c` = 1.1
(iii) `log K_c = 2.2/0.059`
(iv) `log K_c` = 1.1
Conductivity of an electrolytic solution depends on:
(i) nature of electrolyte.
(ii) concentration of electrolyte.
(iii) power of AC source.
(iv) distance between the electrodes.
\[\ce{Λ^0_m H2O}\] is equal to:
(i) \[\ce{Λ^0_m_{(HCl)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaCl)}}}}\]
(ii) \[\ce{Λ^0_m_{(HNO_3)} + \ce{Λ^0_m_{(NaNO_3)} - \ce{Λ^0_m_{(NaOH)}}}}\]
(iii) \[\ce{Λ^0_{(HNO_3)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaNO_3)}}}}\]
(iv) \[\ce{Λ^0_m_{(NH_4OH)} + \ce{Λ^0_m_{(HCl)} - \ce{Λ^0_m_{(NH_4Cl)}}}}\]
What will happen during the electrolysis of aqueous solution of \[\ce{CuSO4}\] by using platinum electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will deposit at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will dissolve at anode.
What will happen during the electrolysis of aqueous solution of CuSO4 in the presence of Cu electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will dissolve at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will deposit at anode.
ConductivityK, is equal to:
(i) `1/R l/A`
(ii) `G^∗/R`
(iii) Λm
(iv) `l/A`
Molar conductivity of ionic solution depends on:
(i) temperature.
(ii) distance between electrodes.
(iii) concentration of electrolytes in solution.
(iv) surface area of electrodes.
For the given cell, \[\ce{Mg | Mg^{2+} || Cu^{2+} | Cu}\]
(i) \[\ce{Mg}\] is cathode
(ii) \[\ce{Cu}\] is cathode
(iii) The cell reaction is \[\ce{Mg^+ Cu^{2+} -> Mg^{2+} + Cu}\]
(iv) \[\ce{Cu}\] is the oxidising agent
Short Answer Type
Can absolute electrode potential of an electrode be measured?
Can `E_(Cell)^Θ` or `∆_rG^Θ` for cell reaction ever be equal to zero?
Under what condition is ECell = 0 or ∆rG = 0?
What does the negative sign in the expression `"E"^Θ ("Zn"^(2+))//("Zn")` = − 0.76 V mean?
Aqueous copper sulphate solution and aqueous silver nitrate solution are electrolysed by 1 ampere current for 10 minutes in separate electrolytic cells. Will the mass of copper and silver deposited on the cathode be same or different? Explain your answer.
Depict the galvanic cell in which the cell reaction is \[\ce{Cu + 2Ag^+ -> 2Ag + Cu^{2+}}\]
Value of standard electrode potential for the oxidation of \[\ce{Cl-}\] ions is more positive than that of water, even then in the electrolysis of aqueous sodium chloride, why is \[\ce{Cl-}\] oxidised at anode instead of water?
What is electrode potential?
Consider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes ‘A’ and ‘B’ in the electrolytic cell?
Why is alternating current used for measuring resistance of an electrolytic solution?
A galvanic cell has electrical potential of 1.1V. If an opposing potential of 1.1V is applied to this cell, what will happen to the cell reaction and current flowing through the cell?
How will the pH of brine (aq. \[\ce{NaCl}\] solution) be affected when it is electrolysed?
Unlike dry cell, the mercury cell has a constant cell potential throughout its useful life. Why?
Solutions of two electrolytes ‘A’ and ‘B’ are diluted. The Λm of ‘B’ increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Justify your answer.
When acidulated water (dil.H2SO4 solution) is electrolysed, will the pH of the solution be affected? Justify your answer.
In an aqueous solution how does specific conductivity of electrolytes change with addition of water?
Which reference electrode is used to measure the electrode potential of other electrodes?
Consider a cell given below:
\[\ce{Cu | Cu^{2+} || Cl^{-} | Cl_{2},Pt}\]
Write the reactions that occur at anode and cathode
Write the Nernst equation for the cell reaction in the Daniel cell. How will the ECell be affected when concentration of Zn2+ ions is increased?
What advantage do the fuel cells have over primary and secondary batteries?
Write the cell reaction of a lead storage battery when it is discharged. How does the density of the electrolyte change when the battery is discharged?
Why on dilution the m Λm of \[\ce{CH3COOH}\] increases very fast, while that of \[\ce{CH3COONa}\] increases gradually?
Matching Type
Match the terms given in Column I with the units given in Column II.
| Column I | Column II |
| (i) Λm | (a) S cm-¹ |
| (ii) ECell | (b) m-¹ |
| (iii) K | (c) S cm2 mol-¹ |
| (iv) G* | (d) V |
Match the terms given in Column I with the items given in Column II.
| Column I | Column II |
| (i) Λm | (a) intensive property |
| (ii) ECell | (b) depends on number of ions/volume |
| (iii) K | (c) extensive property |
| (iv) ∆rGCell | (d) increases with dilution |
Match the items of Column I and Column II.
| Column I | Column II |
| (i) Lead storage battery | (a) maximum efficiency |
| (ii) Mercury cell | (b) prevented by galvanisation |
| (iii) Fuel cell | (c) gives steady potential |
| (iv) Rusting | (d) Pb is anode, PbO2 is cathode |
Match the items of Column I and Column II.
| Column I | Column II |
| (i) K | (a) I × t |
| (ii) Λm | (b) `Λ_m/Λ_m^0` |
| (iii) α | (c) `K/c` |
| (iv) Q | (d) `G^∗/R` |
Match the items of Column I and Column II.
| Column I | Column II |
| (i) Lechlanche cell | (a) cell reaction \[\ce{2H2 + O2 -> 2H2O}\] |
| (ii) Ni–Cd cell | (b) does not involve any ion in solution and is used in hearing aids. |
| (iii) Fuel cell | (c) rechargeable |
| (iv) Mercury cell | (d) reaction at anode, \[\ce{Zn -> Zn^{2+} + 2e^{-}}\] |
| (e) converts energy of combustion into electrical energy |
Match the items of Column I and Column II on the basis of data given below:
`E_("F"_2//"F"^-)^Θ` = 2.87 V, `"E"_(("Li"^(+))//("Li"^-))^Θ` = − 3.5V, `"E"_(("Au"^(3+))//("Au"))^Θ` = 1.4 V, `"E"_(("Br"_(2))//("Br"^-))^Θ` = 1.09 V
| Column I | Column II |
| (i) F2 | (a) metal is the strongest reducing agent |
| (ii) Li | (b) metal ion which is the weakest oxidising agent |
| (iii) Au3+ | (c) non metal which is the best oxidising agent |
| (iv) Br– | (d) unreactive metal |
| (v) Au | (e) anion that can be oxidised by Au3+ |
| (vi) Li+ | (f) anion which is the weakest reducing agent |
| (vii) F– | (g) metal ion which is an oxidising agent |
Assertion and Reason Type Note : In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Cu is less reactive than hydrogen.
Reason: `E_((Cu^(2+))/(Cu))^Θ` is negative.
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Assertion: ECell should have a positive value for the cell to function.
Reason: `"E"_("cathode") < "E"_("anode")`
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Assertion: Conductivity of all electrolytes decreases on dilution.
Reason: On dilution number of ions per unit volume decreases.
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Assertion: Λm for weak electrolytes shows a sharp increase when the electrolytic solution is diluted.
Reason: For weak electrolytes degree of dissociation increases with dilution of solution.
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Assertion: Mercury cell does not give steady potential.
Reason: In the cell reaction, ions are not involved in solution.
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Assertion: Electrolysis of NaCl solution gives chlorine at anode instead of O2.
Reason: Formation of oxygen at anode requires overvoltage.
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Assertion: For measuring resistance of an ionic solution an AC source is used.
Reason: Concentration of ionic solution will change if DC source is used.
Both assertion and reason are true and the reason is the correct explanation of assertion. (ii) Both assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Assertion: Current stops flowing when ECell = 0.
Reason: Equilibrium of the cell reaction is attained.
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Assertion: `"E"_("Ag"^+ //"Ag")` increases with increase in concentration of Ag+ ions.
Reason: `"E"_("Ag"^+ //"Ag")` has a positive value.
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Assertion: Copper sulphate can be stored in zinc vessel.
Reason: Zinc is less reactive than copper.
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Long Answer Type
Consider the figure and answer the following question.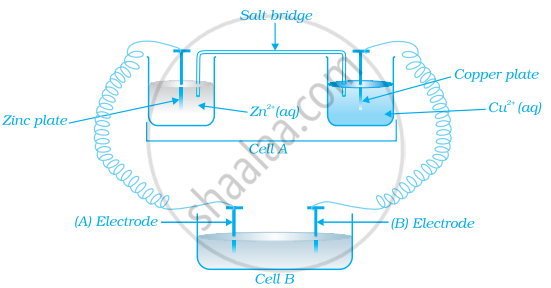
Cell ‘A’ has ECell = 2V and Cell ‘B’ has ECell = 1.1V which of the two cells ‘A’ or ‘B’ will act as an electrolytic cell. Which electrode reactions will occur in this cell?
Consider the figure and answer the following question.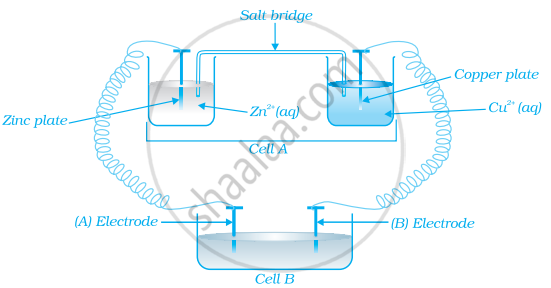
If cell ‘A’ has ECell = 0.5V and cell ‘B’ has ECell = 1.1V then what will be the reactions at anode and cathode?
Consider figure and answer the question to given below.
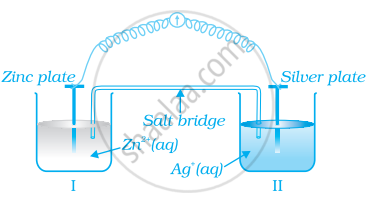
- Redraw the diagram to show the direction of electron flow.
- Is silver plate the anode or cathode?
- What will happen if salt bridge is removed?
- When will the cell stop functioning?
- How will concentration of Zn2+ ions and Ag+ ions be affected when the cell functions?
- How will the concentration of Zn2+ ions and Ag+ ions be affected after the cell becomes ‘dead’?
What is the relationship between Gibbs free energy of the cell reaction in a galvanic cell and the emf of the cell? When will the maximum work be obtained from a galvanic cell?
Solutions for 3: Electrochemistry
![NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 3 - Electrochemistry NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 3 - Electrochemistry - Shaalaa.com](/images/chemistry-english-class-12_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 3 - Electrochemistry
Shaalaa.com has the CBSE Mathematics Chemistry [English] Class 12 CBSE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. NCERT Exemplar solutions for Mathematics Chemistry [English] Class 12 CBSE 3 (Electrochemistry) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. NCERT Exemplar textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] Class 12 chapter 3 Electrochemistry are Electrochemical Cells, Conductance of Electrolytic Solutions - Introduction, Variation of Conductivity and Molar Conductivity with Concentration, Electrolytic Cells and Electrolysis - Introduction, Primary Batteries, Lead Accumulator, Galvanic or Voltaic Cells - Introduction, Nernst Equation - Introduction, Relation Between Gibbs Energy Change and Emf of a Cell, Galvanic Cells - Measurement of Electrode Potential, Equilibrium Constant from Nernst Equation, Electrochemical Cell and Gibbs Energy of the Reaction, Measurement of the Conductivity of Ionic Solutions, Products of Electrolysis, Secondary Batteries, Fuel Cells, Corrosion of Metals, Introduction to Electrochemistry, Faraday’s Law of Induction.
Using NCERT Exemplar Chemistry [English] Class 12 solutions Electrochemistry exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in NCERT Exemplar Solutions are essential questions that can be asked in the final exam. Maximum CBSE Chemistry [English] Class 12 students prefer NCERT Exemplar Textbook Solutions to score more in exams.
Get the free view of Chapter 3, Electrochemistry Chemistry [English] Class 12 additional questions for Mathematics Chemistry [English] Class 12 CBSE, and you can use Shaalaa.com to keep it handy for your exam preparation.