Advertisements
Advertisements
Question
Consider the figure and answer the following question.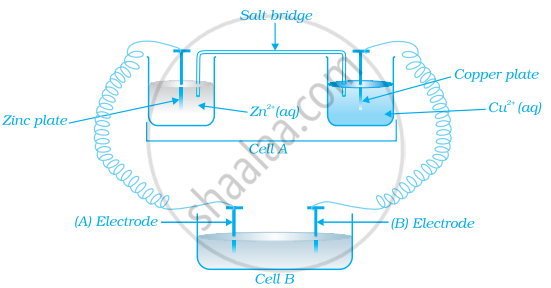
Cell ‘A’ has ECell = 2V and Cell ‘B’ has ECell = 1.1V which of the two cells ‘A’ or ‘B’ will act as an electrolytic cell. Which electrode reactions will occur in this cell?
Solution
Cell ‘B’ will act as electrolytic cell as it has lower emf
∴ The electrode reactions will be:
\[\ce{Zn^{2+} + 2e^{-} -> Zn}\] at cathode
\[\ce{Cu -> Cu^{2+} + 2e^{-}}\] at anode
APPEARS IN
RELATED QUESTIONS
On calculating the strength of current in amperes if a charge of 840C (coulomb) passes through an electrolyte in 7 minutes, it will be
- 1
- 2
- 3
- 4
Number of faradays of electricity required to liberate 12 g of hydrogen is:
If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Suggest a list of metals that are extracted electrolytically.
How much charge is required for the following reduction:
1 mol of \[\ce{Al^{3+}}\] to \[\ce{Al}\]?
State second law of electrolysis
What will happen during the electrolysis of aqueous solution of CuSO4 in the presence of Cu electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will dissolve at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will deposit at anode.
Aqueous copper sulphate solution and aqueous silver nitrate solution are electrolysed by 1 ampere current for 10 minutes in separate electrolytic cells. Will the mass of copper and silver deposited on the cathode be same or different? Explain your answer.
Given `1/a` = 0.5 CM–1, R = 50 ohm, N = 1.0 then equivalent conductance of electrolytic cell is
On passing electricity through nitrobenzene solution, it is converted into azobenzene. The mass of azobenzene is ______ mg, if the same quantity of electricity produces oxygen just sufficient to burn 96 mg of fullerene (C60)·
