Advertisements
Advertisements
Question
If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Solution
I = 0.5 A
t = 2 hours = 2 × 60 × 60 s = 7200 s
Thus, Q = It
= 0.5 A × 7200 s
= 3600 C
We know that 96487C = 6.023 × 1023 number of electrons
Then
`3600 "C" = (6.023 xx 10^(23) xx 3600)/9648` number of electrons
= 2.25 × 1022 number of electrons
Hence, 2.25 × 1022 number of electrons will flow through the wire.
APPEARS IN
RELATED QUESTIONS
On calculating the strength of current in amperes if a charge of 840C (coulomb) passes through an electrolyte in 7 minutes, it will be
- 1
- 2
- 3
- 4
96500 coulombs correspond to the charge on how many electrons?
Number of faradays of electricity required to liberate 12 g of hydrogen is:
How much charge is required for the following reduction:
1 mol of \[\ce{Al^{3+}}\] to \[\ce{Al}\]?
How much charge is required for the following reduction:
1 mol of \[\ce{Cu^{2+}}\] to \[\ce{Cu}\]?
What is the ratio of volumes of H2 and O2 liberated during electrolysis of acidified water?
(A) 1 : 2
(B) 2 : 1
(C) 1 : 8
(D) 8 : 1
Calculate the mass of Ag deposited at cathode when a current of 2 amperes was passed through a solution of AgNO3 for 15 minutes.
(Given : Molar mass of Ag = 108 g mol−1 lF = 96500 C mol−1)
Following reactions occur at cathode during the electrolysis of aqueous copper(II) chloride solution :
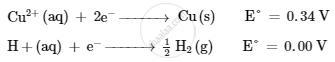
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why ?
Electrolytic cell uses electrical energy to bring about ____________.
According to Faraday’s First Law of Electrolysis, the amount of chemical reaction which occurs at any electrode during electrolysis by a current is proportional to the ____________.
Assertion: Electrolysis of NaCl solution gives chlorine at anode instead of O2.
Reason: Formation of oxygen at anode requires overvoltage.
Consider the figure and answer the following question.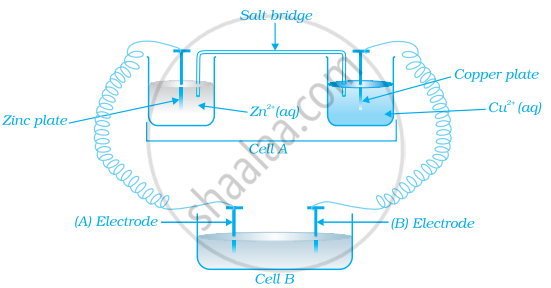
Cell ‘A’ has ECell = 2V and Cell ‘B’ has ECell = 1.1V which of the two cells ‘A’ or ‘B’ will act as an electrolytic cell. Which electrode reactions will occur in this cell?
Time Required to deposite one millimole of aluminium metal by the passage of 9.65 ampere through aqueous solution of aluminium is
Given `1/a` = 0.5 CM–1, R = 50 ohm, N = 1.0 then equivalent conductance of electrolytic cell is
The quantity of electricity needed to separately electrolyse 1 M solution of ZnSO4, AlCl3, and AgNO3 completely is in the ratio of ______.
Through an aqueous solution of an unknown salt of metal M (M = 200 g/mol) a current of 1.93 A is passed for 50 min. If 4 g of metal is produced at cathode. The charge on metal ion in solution is ______.
A current of 4 amp was passed for 2 hours through a solution of copper sulphate when 5.0 g of copper was deposited. The current efficiency is ______% (Cu = 63.5).
Assertion (A): During electrolysis of aqueous copper sulphate solution using copper electrodes hydrogen gas is released at the cathode.
Reason (R): The electrode potential of Cu2+/Cu is greater than that of H+/H2.
Select the most appropriate answer from the options given below:
How much electricity in terms of Faraday is required to produce 40.0 g of \[\ce{Al}\] from molten \[\ce{Al2O3}\]?
(Given: Molar mass of Aluminium is 27 g mol−1.)
