Advertisements
Advertisements
Question
The molar conductivity of 0.025 mol L−1 methanoic acid is 46.1 S cm2 mol−1. Calculate its degree of dissociation and dissociation constant. Given \[\ce{λ^0_{(H^+)}}\] = 349.6 S cm2 mol−1 and \[\ce{λ^0_{(HCOO^-)}}\] = 54.6 S cm2 mol−1.
Solution
\[\ce{∧^0_{{m}(HCOOH)} = λ^0_{(H^+)} + λ^0_{(HCOO^-)}}\]
= 349.6 + 54.6
= 404.2 S cm2 mol−1
Given: \[\ce{∧_{{m}(HCOOH)}}\] = 46.1 S cm2 mol−1
Degree of dissociation, α = `∧_"m"/(∧_"m"^0)`
= `(46.1 "S cm"^2 "mol"^-1)/(404.2 "S cm"^2 "mol"^-1)`
= 0.114
\[\ce{HCOOH ⇌ HCOO^- + H^+}\]
| Initial concentration | c mol L−1 | 0 | 0 |
| Concentration at equilibrium | c(1 − α) | cα | cα |
∴ Kα = `("c"α . "c"α)/("c"(1 - α))`
= `("c"α^2)/(1 - α)`
= `(0.025 xx (0.114)^2)/(1 - 0.114)`
= 3.67 × 10−4
APPEARS IN
RELATED QUESTIONS
Define "Molar conductivity".
Resistance of conductivity cell filled with 0.1 M KCl solution is 100 ohms. If the resistance of the same cell when filled with 0.02 M KCl solution is 520 ohms, calculate the conductivity and molar conductivity of 0.02 M KCl solution. [Given: Conductivity of 0.1 M KCl solution is 1.29 S m-1 .]
State Kohlrausch’s law of independent migration of ions.
The molar conductivity of cation and anion of salt BA are 180 and 220 mhos respectively. The molar conductivity of salt BA at infinite dilution is_____________ .
(a) 90 mhos.cm2
(b) 110 mhos.cm2.mol-1
(c) 200 mhos.cm2.mol-1
(d) 400 mhos.cm2.mol-1
State Kohlrausch Law
Why does the conductivity of a solution decrease with dilution?
The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
| Concentration/M | 0.001 | 0.010 | 0.020 | 0.050 | 0.100 |
| 102 × κ/S m−1 | 1.237 | 11.85 | 23.15 | 55.53 | 106.74 |
Calculate `∧_"m"`for all concentrations and draw a plot between `∧_"m"`and `"c"^(1/2)`. Find the value of `∧_"m"^0`.
Write mathematical expression of molar conductivity of the given solution at infinite dilution.
Molar conductivity of ionic solution depends on:
(i) temperature.
(ii) distance between electrodes.
(iii) concentration of electrolytes in solution.
(iv) surface area of electrodes.
Solutions of two electrolytes ‘A’ and ‘B’ are diluted. The Λm of ‘B’ increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Justify your answer.
Match the items of Column I and Column II on the basis of data given below:
`E_("F"_2//"F"^-)^Θ` = 2.87 V, `"E"_(("Li"^(+))//("Li"^-))^Θ` = − 3.5V, `"E"_(("Au"^(3+))//("Au"))^Θ` = 1.4 V, `"E"_(("Br"_(2))//("Br"^-))^Θ` = 1.09 V
| Column I | Column II |
| (i) F2 | (a) metal is the strongest reducing agent |
| (ii) Li | (b) metal ion which is the weakest oxidising agent |
| (iii) Au3+ | (c) non metal which is the best oxidising agent |
| (iv) Br– | (d) unreactive metal |
| (v) Au | (e) anion that can be oxidised by Au3+ |
| (vi) Li+ | (f) anion which is the weakest reducing agent |
| (vii) F– | (g) metal ion which is an oxidising agent |
Consider figure and answer the question to given below.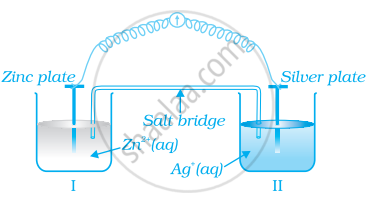
How will the concentration of Zn2+ ions and Ag+ ions be affected after the cell becomes ‘dead’?
Which of the following increases with the increase in the concentration of the solution?
The molar conductance of \[\ce{NaCl, HCl}\] and \[\ce{CH3COONa}\] at infinite dilution are 126.45, 426.16 and 91.0 S cm2 mol−1 respectively. The molar conductance of \[\ce{CH3COOH}\] at infinite dilution is. Choose the right option for your answer.
The solubility of Co2[Fe(CN)6] in water at 25°C from the following data:
Conductivity of saturated solution of Co2[Fe(CN)6] = 2.06 × 10−6 ohm−1 cm−1 and that of water = 4.1 × 10−7 ohm−1 cm−1. The ionic molar conductivities of Co2+ and [Fe(CN)6]4− are 86 and 444 ohm−1 cm2 mol−1 respectively, is ______ × 10−6 mol/L.
The variation of molar conductivity with concentration of an electrolyte (X) m aqueous solution is shown in the given figure.
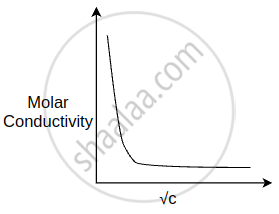
The electrolyte X is ______.
Assertion (A): Molar conductivity decreases with increase in concentration.
Reason (R): When concentration approaches zero, the molar conductivity is known as limiting molar conductivity.
The resistance of a conductivity cell with a 0.1 M KCl solution is 200 ohm. When the same cell is filled with a 0.02 M NaCl solution, the resistance is 1100 ohm. If the conductivity of 0.1 M KCl solution is 0.0129 ohm-1 cm-1, calculate the cell constant and molar conductivity of 0.02 M NaCl solution.
