Advertisements
Advertisements
Question
Write mathematical expression of molar conductivity of the given solution at infinite dilution.
Solution
`lambda^@ = lambda_+^@ + lambda_-^@`
where,
`lambda^@` is molar conductivity of electrolytic solution
`lambda_+^@` is molar conductivity of cation
`lambda_-^@` is molar conductivity of anion
APPEARS IN
RELATED QUESTIONS
State Kohlrausch’s law of independent migration of ions.
Why does the conductivity of a solution decrease with dilution?
How can you determine limiting molar conductivity, 0 m for strong electrolyte and weak electrolyte?
The S.I. unit of cell constant for conductivity cell is __________.
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2), following curves are obtained for two electrolytes A and B:
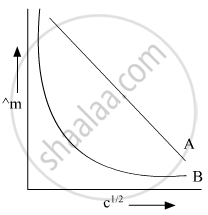
Answer the following:
(i) Predict the nature of electrolytes A and B.
(ii) What happens on extrapolation of ∧m to concentration approaching zero for electrolytes A and B?
Kohlrausch law of independent migration of ions states ____________.
Which of the statements about solutions of electrolytes is not correct?
\[\ce{Λ^0_m}_{(NH_4OH)}\] is equal to ______.
\[\ce{Λ^0_m H2O}\] is equal to:
(i) \[\ce{Λ^0_m_{(HCl)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaCl)}}}}\]
(ii) \[\ce{Λ^0_m_{(HNO_3)} + \ce{Λ^0_m_{(NaNO_3)} - \ce{Λ^0_m_{(NaOH)}}}}\]
(iii) \[\ce{Λ^0_{(HNO_3)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaNO_3)}}}}\]
(iv) \[\ce{Λ^0_m_{(NH_4OH)} + \ce{Λ^0_m_{(HCl)} - \ce{Λ^0_m_{(NH_4Cl)}}}}\]
Molar conductivity of ionic solution depends on:
(i) temperature.
(ii) distance between electrodes.
(iii) concentration of electrolytes in solution.
(iv) surface area of electrodes.
Why on dilution the m Λm of \[\ce{CH3COOH}\] increases very fast, while that of \[\ce{CH3COONa}\] increases gradually?
Match the items of Column I and Column II on the basis of data given below:
`E_("F"_2//"F"^-)^Θ` = 2.87 V, `"E"_(("Li"^(+))//("Li"^-))^Θ` = − 3.5V, `"E"_(("Au"^(3+))//("Au"))^Θ` = 1.4 V, `"E"_(("Br"_(2))//("Br"^-))^Θ` = 1.09 V
| Column I | Column II |
| (i) F2 | (a) metal is the strongest reducing agent |
| (ii) Li | (b) metal ion which is the weakest oxidising agent |
| (iii) Au3+ | (c) non metal which is the best oxidising agent |
| (iv) Br– | (d) unreactive metal |
| (v) Au | (e) anion that can be oxidised by Au3+ |
| (vi) Li+ | (f) anion which is the weakest reducing agent |
| (vii) F– | (g) metal ion which is an oxidising agent |
Assertion: Λm for weak electrolytes shows a sharp increase when the electrolytic solution is diluted.
Reason: For weak electrolytes degree of dissociation increases with dilution of solution.
An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to :-
Which of the following increases with the increase in the concentration of the solution?
Molar conductivity of substance “A” is 5.9 × 103 S/m and “B” is 1 × 10–16 S/m. Which of the two is most likely to be copper metal and why?
The solubility of Co2[Fe(CN)6] in water at 25°C from the following data:
Conductivity of saturated solution of Co2[Fe(CN)6] = 2.06 × 10−6 ohm−1 cm−1 and that of water = 4.1 × 10−7 ohm−1 cm−1. The ionic molar conductivities of Co2+ and [Fe(CN)6]4− are 86 and 444 ohm−1 cm2 mol−1 respectively, is ______ × 10−6 mol/L.
The variation of molar conductivity with concentration of an electrolyte (X) m aqueous solution is shown in the given figure.
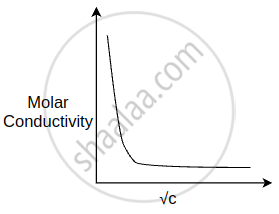
The electrolyte X is ______.
Which of the following solutions of KCl will have the highest value of molar conductivity?
Conductivity of 2 × 10−3 M methanoic acid is 8 × 10−5 S cm−1. Calculate its molar conductivity and degree of dissociation if `∧_"m"^0` for methanoic acid, is 404 S cm2 mol−3.
Which of the following solutions will have the highest conductivity at 298 K?
The specific conductance of 2.5 × 10-4 M formic acid is 5.25 × 10-5 ohm-1 cm-1. Calculate its molar conductivity and degree of dissociation.
Given `λ°_("H"^+)` = 349.5 ohm-1 cm2 mol-1 and
`λ°_("HCOO"^-) = 50.5 " ohm"^-1 "cm"^2 "mol"^-1`
The resistance of a conductivity cell with a 0.1 M KCl solution is 200 ohm. When the same cell is filled with a 0.02 M NaCl solution, the resistance is 1100 ohm. If the conductivity of 0.1 M KCl solution is 0.0129 ohm-1 cm-1, calculate the cell constant and molar conductivity of 0.02 M NaCl solution.
The solution of two electrolytes A and B are diluted. ^m of B increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Give a reason.
Suggest a way to determine the `∧_"m"^∘`value of water.
Discuss the variation of conductivity and molar conductivity with concentration.
