Advertisements
Advertisements
Question
The S.I. unit of cell constant for conductivity cell is __________.
Options
m-1
S m-2
cm-2
S dm2 mol-1
Solution
The S.I. unit of cell constant for conductivity cell is m-1.
APPEARS IN
RELATED QUESTIONS
State Kohlrausch Law
The conductivity of 0.20 mol L−1 solution of KCl is 2.48 × 10−2 S cm−1. Calculate its molar conductivity and degree of dissociation (α). Given λ0 (K+) = 73.5 S cm2 mol−1 and λ0 (C1−) = 76.5 S cm2 mol−1.
Why does the conductivity of a solution decrease with dilution?
Define the following terms: Molar conductivity (⋀m)
Conductivity of 0.00241 M acetic acid is 7.896 × 10−5 S cm−1. Calculate its molar conductivity and if `∧_"m"^0` for acetic acid is 390.5 S cm2 mol−1, what is its dissociation constant?
The conductivity of 0.02M AgNO3 at 25°C is 2.428 x 10-3 Ω-1 cm-1. What is its molar
conductivity?
Calculate the degree of dissociation (α) of acetic acid if its molar conductivity (Λm) is 39.05 S cm2 mol−1.
Given λ°(H+) = 349.6 S cm2 mol−1 and λ°(CH3COO−) = 40.9 S cm2 mol−1
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2) following curves are obtained for two electrolytes A and B : 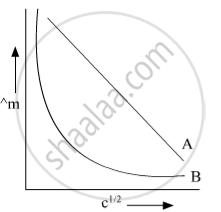
Answer the following:
(i) predict the nature of electrolytes A and B.
(ii) What happens on the extrapolation of ∧m to concentration approaching for electrolytes A and B?
Kohlrausch law of independent migration of ions states ____________.
\[\ce{Λ^0_m}_{(NH_4OH)}\] is equal to ______.
\[\ce{Λ^0_m H2O}\] is equal to:
(i) \[\ce{Λ^0_m_{(HCl)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaCl)}}}}\]
(ii) \[\ce{Λ^0_m_{(HNO_3)} + \ce{Λ^0_m_{(NaNO_3)} - \ce{Λ^0_m_{(NaOH)}}}}\]
(iii) \[\ce{Λ^0_{(HNO_3)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaNO_3)}}}}\]
(iv) \[\ce{Λ^0_m_{(NH_4OH)} + \ce{Λ^0_m_{(HCl)} - \ce{Λ^0_m_{(NH_4Cl)}}}}\]
Molar conductivity of ionic solution depends on:
(i) temperature.
(ii) distance between electrodes.
(iii) concentration of electrolytes in solution.
(iv) surface area of electrodes.
Solutions of two electrolytes ‘A’ and ‘B’ are diluted. The Λm of ‘B’ increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Justify your answer. Graphically show the behavior of ‘A’ and ‘B’.
Which of the following increases with the increase in the concentration of the solution?
The molar conductance of \[\ce{NaCl, HCl}\] and \[\ce{CH3COONa}\] at infinite dilution are 126.45, 426.16 and 91.0 S cm2 mol−1 respectively. The molar conductance of \[\ce{CH3COOH}\] at infinite dilution is. Choose the right option for your answer.
The molar conductivity of 0.007 M acetic acid is 20 S cm2 mol−1. What is the dissociation constant of acetic acid? Choose the correct option.
`[(Λ_("H"^+)^ο = 350 "S" "cm"^2 "mol"^-1), (Λ_("CH"_3"COO"^-)^ο = 50 "S" "cm"^2 "mol"^-1)]`
The molar conductance of NaCl, HCl, and CH3COONa at infinite dilution are 126.45, 426.16, and 91.0 S cm2 mol−1 respectively. The molar conductance of CH3COOH at infinite dilution is. Choose the right option for your answer.
The molar conductivity of CH3COOH at infinite dilution is 390 Scm2/mol. Using the graph and given information, the molar conductivity of CH3COOK will be:
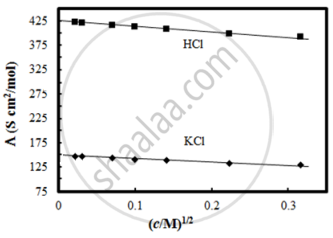
Given below are two statements:
Statements I: The limiting molar conductivity of KCl (strong electrolyte) is higher compared to that of CH3COOH (weak electrolyte).
Statement II: Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
The solubility of Co2[Fe(CN)6] in water at 25°C from the following data:
Conductivity of saturated solution of Co2[Fe(CN)6] = 2.06 × 10−6 ohm−1 cm−1 and that of water = 4.1 × 10−7 ohm−1 cm−1. The ionic molar conductivities of Co2+ and [Fe(CN)6]4− are 86 and 444 ohm−1 cm2 mol−1 respectively, is ______ × 10−6 mol/L.
Which of the following solutions of KCl will have the highest value of molar conductivity?
Which of the following solutions will have the highest conductivity at 298 K?
The resistance of a conductivity cell with a 0.1 M KCl solution is 200 ohm. When the same cell is filled with a 0.02 M NaCl solution, the resistance is 1100 ohm. If the conductivity of 0.1 M KCl solution is 0.0129 ohm-1 cm-1, calculate the cell constant and molar conductivity of 0.02 M NaCl solution.
Discuss the variation of conductivity and molar conductivity with concentration.
