Advertisements
Advertisements
प्रश्न
The S.I. unit of cell constant for conductivity cell is __________.
पर्याय
m-1
S m-2
cm-2
S dm2 mol-1
उत्तर
The S.I. unit of cell constant for conductivity cell is m-1.
APPEARS IN
संबंधित प्रश्न
Define "Molar conductivity".
Resistance of conductivity cell filled with 0.1 M KCl solution is 100 ohms. If the resistance of the same cell when filled with 0.02 M KCl solution is 520 ohms, calculate the conductivity and molar conductivity of 0.02 M KCl solution. [Given: Conductivity of 0.1 M KCl solution is 1.29 S m-1 .]
State Kohlrausch Law
The conductivity of 0.001 mol L-1 solution of CH3COOH is 3.905× 10-5 S cm-1. Calculate its molar conductivity and degree of dissociation (α) Given λ°(H+)= 349.6 S cm2 mol-1 and λ°(CH3COO)= 40.9S cm2mol-1.
Define limiting molar conductivity.
Define the following terms: Molar conductivity (⋀m)
The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
| Concentration/M | 0.001 | 0.010 | 0.020 | 0.050 | 0.100 |
| 102 × κ/S m−1 | 1.237 | 11.85 | 23.15 | 55.53 | 106.74 |
Calculate `∧_"m"`for all concentrations and draw a plot between `∧_"m"`and `"c"^(1/2)`. Find the value of `∧_"m"^0`.
How can you determine limiting molar conductivity, 0 m for strong electrolyte and weak electrolyte?
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2) following curves are obtained for two electrolytes A and B : 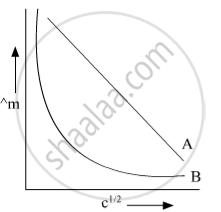
Answer the following:
(i) predict the nature of electrolytes A and B.
(ii) What happens on the extrapolation of ∧m to concentration approaching for electrolytes A and B?
Conductivity always decreases with decrease in concentration both, for weak and strong electrolytes because of the fact that ____________.
\[\ce{Λ^0_m H2O}\] is equal to:
(i) \[\ce{Λ^0_m_{(HCl)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaCl)}}}}\]
(ii) \[\ce{Λ^0_m_{(HNO_3)} + \ce{Λ^0_m_{(NaNO_3)} - \ce{Λ^0_m_{(NaOH)}}}}\]
(iii) \[\ce{Λ^0_{(HNO_3)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaNO_3)}}}}\]
(iv) \[\ce{Λ^0_m_{(NH_4OH)} + \ce{Λ^0_m_{(HCl)} - \ce{Λ^0_m_{(NH_4Cl)}}}}\]
When acidulated water (dil.H2SO4 solution) is electrolysed, will the pH of the solution be affected? Justify your answer.
Match the items of Column I and Column II on the basis of data given below:
`E_("F"_2//"F"^-)^Θ` = 2.87 V, `"E"_(("Li"^(+))//("Li"^-))^Θ` = − 3.5V, `"E"_(("Au"^(3+))//("Au"))^Θ` = 1.4 V, `"E"_(("Br"_(2))//("Br"^-))^Θ` = 1.09 V
| Column I | Column II |
| (i) F2 | (a) metal is the strongest reducing agent |
| (ii) Li | (b) metal ion which is the weakest oxidising agent |
| (iii) Au3+ | (c) non metal which is the best oxidising agent |
| (iv) Br– | (d) unreactive metal |
| (v) Au | (e) anion that can be oxidised by Au3+ |
| (vi) Li+ | (f) anion which is the weakest reducing agent |
| (vii) F– | (g) metal ion which is an oxidising agent |
Assertion: Λm for weak electrolytes shows a sharp increase when the electrolytic solution is diluted.
Reason: For weak electrolytes degree of dissociation increases with dilution of solution.
Assertion: `"E"_("Ag"^+ //"Ag")` increases with increase in concentration of Ag+ ions.
Reason: `"E"_("Ag"^+ //"Ag")` has a positive value.
An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to :-
Which of the following increases with the increase in the concentration of the solution?
The molar conductivity of 0.007 M acetic acid is 20 S cm2 mol−1. What is the dissociation constant of acetic acid? Choose the correct option.
`[(Λ_("H"^+)^ο = 350 "S" "cm"^2 "mol"^-1), (Λ_("CH"_3"COO"^-)^ο = 50 "S" "cm"^2 "mol"^-1)]`
The molar conductance of NaCl, HCl, and CH3COONa at infinite dilution are 126.45, 426.16, and 91.0 S cm2 mol−1 respectively. The molar conductance of CH3COOH at infinite dilution is. Choose the right option for your answer.
Which of the following solutions of KCl will have the highest value of molar conductivity?
Assertion (A) : Conductivity decreases with decrease in concentration of electrolyte.
Reason (R) : Number of ions per unit volume that carry the current in a solution decreases on dilution.
The unit of molar conductivity is ______.
Which of the following solutions will have the highest conductivity at 298 K?
The specific conductance of 2.5 × 10-4 M formic acid is 5.25 × 10-5 ohm-1 cm-1. Calculate its molar conductivity and degree of dissociation.
Given `λ°_("H"^+)` = 349.5 ohm-1 cm2 mol-1 and
`λ°_("HCOO"^-) = 50.5 " ohm"^-1 "cm"^2 "mol"^-1`
The solution of two electrolytes A and B are diluted. ^m of B increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Give a reason.
