Advertisements
Advertisements
प्रश्न
\[\ce{Λ^0_m H2O}\] is equal to:
(i) \[\ce{Λ^0_m_{(HCl)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaCl)}}}}\]
(ii) \[\ce{Λ^0_m_{(HNO_3)} + \ce{Λ^0_m_{(NaNO_3)} - \ce{Λ^0_m_{(NaOH)}}}}\]
(iii) \[\ce{Λ^0_{(HNO_3)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaNO_3)}}}}\]
(iv) \[\ce{Λ^0_m_{(NH_4OH)} + \ce{Λ^0_m_{(HCl)} - \ce{Λ^0_m_{(NH_4Cl)}}}}\]
उत्तर
(i) \[\ce{Λ^0_m_{(HCl)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaCl)}}}}\]
(iv) \[\ce{Λ^0_m_{(NH_4OH)} + \ce{Λ^0_m_{(HCl)} - \ce{Λ^0_m_{(NH_4Cl)}}}}\]
Explanation:
\[\ce{Λ^0_m_{(H_2O)} = \ce{Λ^0_m_{(HCl)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaCl)}}}}}\]
\[\ce{Λ^0_{m(H^+)} + \ce{Λ^0_{m(OH^-)} = \ce{Λ^0_{m(H^+)} + \ce{Λ^0_{m(Cl^-)} + \ce{Λ^0_{m(Na^+)} + \ce{Λ^0_{m(OH^-)} - \ce{Λ^0_{m(Na^+)} - \ce{Λ^0_{m(Cl^-)}}}}}}}}}\]
\[\ce{Λ^0_m_{(HNO_3)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaNO_3)} = \ce{Λ^0_m_{(H_2O)}}}}}\]
\[\ce{Λ^0_{m(H^+)} + \ce{Λ^0_{m(NO_3^-)} + \ce{Λ^0_{m(Na^+)} - \ce{Λ^0_{m(OH^-)} + \ce{Λ^0_{m(Na^+)} + \ce{Λ^0_{m(NO_3^-)} = \ce{Λ^0_{m(H^+)} - \ce{Λ^0_{m(OH^-)}}}}}}}}}\]
\[\ce{Λ^0_m_{(NH_4OH)} + \ce{Λ^0_m_{(HCl)} - \ce{Λ^0_m_{(NH_4Cl)} = \ce{Λ^0_m_{(H_2O)}}}}}\]
However, the sum of molar conductivities of constituent ions gives the molar conductivity of water but here \[\ce{NH4OH}\] is a weak electrolyte of which complete decomposition is not possible.
APPEARS IN
संबंधित प्रश्न
The conductivity of 0.02M AgNO3 at 25°C is 2.428 x 10-3 Ω-1 cm-1. What is its molar
conductivity?
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2), following curves are obtained for two electrolytes A and B:
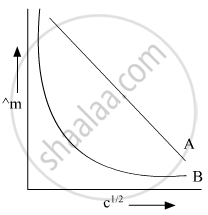
Answer the following:
(i) Predict the nature of electrolytes A and B.
(ii) What happens on extrapolation of ∧m to concentration approaching zero for electrolytes A and B?
Conductivity always decreases with decrease in concentration both, for weak and strong electrolytes because of the fact that ____________.
Kohlrausch law of independent migration of ions states ____________.
Molar conductivity of ionic solution depends on:
(i) temperature.
(ii) distance between electrodes.
(iii) concentration of electrolytes in solution.
(iv) surface area of electrodes.
Why on dilution the m Λm of \[\ce{CH3COOH}\] increases very fast, while that of \[\ce{CH3COONa}\] increases gradually?
Assertion: Copper sulphate can be stored in zinc vessel.
Reason: Zinc is less reactive than copper.
Which of the following increases with the increase in the concentration of the solution?
Molar conductivity of substance “A” is 5.9 × 103 S/m and “B” is 1 × 10–16 S/m. Which of the two is most likely to be copper metal and why?
The solution of two electrolytes A and B are diluted. ^m of B increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Give a reason.
