Advertisements
Advertisements
Question
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2) following curves are obtained for two electrolytes A and B : 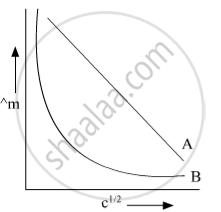
Answer the following:
(i) predict the nature of electrolytes A and B.
(ii) What happens on the extrapolation of ∧m to concentration approaching for electrolytes A and B?
Solution
Electrolyte A is strong electrolyte & Electrolyte B is weak electrolyte On extrapolation of Λm to concentration approaching zero for strong electrolytes, we get the value of Λ=m i.e. molar conductance at infinite dilution In the case of weak electrolytes, Λm increases steeply on dilution. Therefore, Λ=m cannot be obtained by extrapolation.
APPEARS IN
RELATED QUESTIONS
Conductivity of 0.00241 M acetic acid is 7.896 × 10−5 S cm−1. Calculate its molar conductivity and if `∧_"m"^0` for acetic acid is 390.5 S cm2 mol−1, what is its dissociation constant?
Calculate the degree of dissociation (α) of acetic acid if its molar conductivity (Λm) is 39.05 S cm2 mol−1.
Given λ°(H+) = 349.6 S cm2 mol−1 and λ°(CH3COO−) = 40.9 S cm2 mol−1
How can you determine limiting molar conductivity, 0 m for strong electrolyte and weak electrolyte?
Conductivity always decreases with decrease in concentration both, for weak and strong electrolytes because of the fact that ____________.
Kohlrausch law of independent migration of ions states ____________.
When acidulated water (dil.H2SO4 solution) is electrolysed, will the pH of the solution be affected? Justify your answer.
Match the items of Column I and Column II on the basis of data given below:
`E_("F"_2//"F"^-)^Θ` = 2.87 V, `"E"_(("Li"^(+))//("Li"^-))^Θ` = − 3.5V, `"E"_(("Au"^(3+))//("Au"))^Θ` = 1.4 V, `"E"_(("Br"_(2))//("Br"^-))^Θ` = 1.09 V
| Column I | Column II |
| (i) F2 | (a) metal is the strongest reducing agent |
| (ii) Li | (b) metal ion which is the weakest oxidising agent |
| (iii) Au3+ | (c) non metal which is the best oxidising agent |
| (iv) Br– | (d) unreactive metal |
| (v) Au | (e) anion that can be oxidised by Au3+ |
| (vi) Li+ | (f) anion which is the weakest reducing agent |
| (vii) F– | (g) metal ion which is an oxidising agent |
Which of the following halogen acids is the strongest reducing agent?
The solution of two electrolytes A and B are diluted. ^m of B increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Give a reason.
Discuss the variation of conductivity and molar conductivity with concentration.
