Advertisements
Advertisements
Question
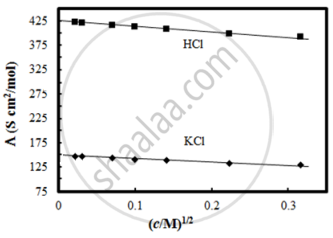
The molar conductivity of CH3COOH at infinite dilution is 390 Scm2/mol. Using the graph and given information, the molar conductivity of CH3COOK will be:
Options
100 Scm2/mol
115 Scm2/mol
150 Scm2/mol
125 Scm2/mol
Solution
115 Scm2/mol
Explanation:
Λ°CH3COOK = Λ°CH3COOH + Λ°KCl – Λ°HCl
= 390 + 150 – 425
= 115 Scm2/mol
APPEARS IN
RELATED QUESTIONS
State Kohlrausch’s law of independent migration of ions.
The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm−1. Calculate its molar conductivity.
Define limiting molar conductivity.
Why does the conductivity of a solution decrease with dilution?
10.0 grams of caustic soda when dissolved in 250 cm3 of water, the resultant gram molarity of solution is _______.
(A) 0.25 M
(B) 0.5 M
(C) 1.0 M
(D) 0.1 M
The conductivity of 0.02M AgNO3 at 25°C is 2.428 x 10-3 Ω-1 cm-1. What is its molar
conductivity?
Why on dilution the m Λm of \[\ce{CH3COOH}\] increases very fast, while that of \[\ce{CH3COONa}\] increases gradually?
Which of the following halogen acids is the strongest reducing agent?
The molar conductance of NaCl, HCl, and CH3COONa at infinite dilution are 126.45, 426.16, and 91.0 S cm2 mol−1 respectively. The molar conductance of CH3COOH at infinite dilution is. Choose the right option for your answer.
The solution of two electrolytes A and B are diluted. ^m of B increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Give a reason.
