Advertisements
Advertisements
Question
Why on dilution the m Λm of \[\ce{CH3COOH}\] increases very fast, while that of \[\ce{CH3COONa}\] increases gradually?
Solution
In the case of \[\ce{CH3COOH}\], which is a weak electrolyte, the number of ions increase on dilution due to an increase in degree of dissociation.
\[\ce{CH3COOH + H2O ⇌ CH3COO^{-} (aq) + H3O^{+}}\]
In the case of strong electrolyte such as \[\ce{CH3COONa}\], the number of ions remains the same but the interionic attraction decreases.
RELATED QUESTIONS
The conductivity of 0.001 mol L-1 solution of CH3COOH is 3.905× 10-5 S cm-1. Calculate its molar conductivity and degree of dissociation (α) Given λ°(H+)= 349.6 S cm2 mol-1 and λ°(CH3COO)= 40.9S cm2mol-1.
Define limiting molar conductivity.
Define the following terms :
Limiting molar conductivity
Which of the statements about solutions of electrolytes is not correct?
Assertion: Λm for weak electrolytes shows a sharp increase when the electrolytic solution is diluted.
Reason: For weak electrolytes degree of dissociation increases with dilution of solution.
The molar conductivity of CH3COOH at infinite dilution is 390 Scm2/mol. Using the graph and given information, the molar conductivity of CH3COOK will be:
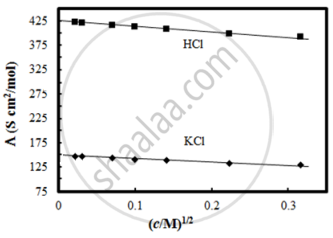
Given below are two statements:
Statements I: The limiting molar conductivity of KCl (strong electrolyte) is higher compared to that of CH3COOH (weak electrolyte).
Statement II: Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
The solubility of Co2[Fe(CN)6] in water at 25°C from the following data:
Conductivity of saturated solution of Co2[Fe(CN)6] = 2.06 × 10−6 ohm−1 cm−1 and that of water = 4.1 × 10−7 ohm−1 cm−1. The ionic molar conductivities of Co2+ and [Fe(CN)6]4− are 86 and 444 ohm−1 cm2 mol−1 respectively, is ______ × 10−6 mol/L.
Which of the following solutions will have the highest conductivity at 298 K?
Discuss the variation of conductivity and molar conductivity with concentration.
