Advertisements
Advertisements
Question
Match the terms given in Column I with the units given in Column II.
| Column I | Column II |
| (i) Λm | (a) S cm-¹ |
| (ii) ECell | (b) m-¹ |
| (iii) K | (c) S cm2 mol-¹ |
| (iv) G* | (d) V |
Solution
| Column I | Column II |
| (i) Λm | (c) S cm2 mol-¹ |
| (ii) ECell | (d) V |
| (iii) K | (a) S cm-¹ |
| (iv) G* | (b) m-¹ |
APPEARS IN
RELATED QUESTIONS
Assertion: pure iron when heated in dry air is converted with a layer of rust.
Reason: Rust has the compositionFe3O4.
Define cathode
A copper electrode is dipped in 0.1 M copper sulphate solution at 25°C. Calculate the electrode potential of copper.
[Given: \[\ce{E^0_{{Cu^{2+}|Cu}}}\] = 0.34 V]
Which of the following statement is not correct about an inert electrode in a cell?
Use the data given in below find out which option the order of reducing power is correct.
`"E"_("Cr"_2"O"_7^(2-)//"Cr"^(3+))^⊖`= 1.33 V `"E"_("Cl"_2//"Cl"^-)^⊖` = 1.36 V
`"E"_("MnO"_4^-//"Mn"^(2+))^⊖` = 1.51 V `"E"_("Cr"^(3+)//"Cr")^⊖` = - 0.74 V
What is electrode potential?
The electrochemical cell stops working after some time because
In a Daniel cell, ______.
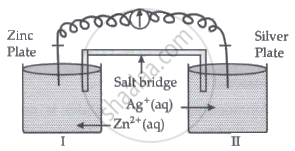
Read the passage given below and answer the questions that follow:
|
Oxidation-reduction reactions are commonly known as redox reactions. They involve transfer of electrons from one species to another. In a spontaneous reaction, energy is released which can be used to do useful work. The reaction is split into two half-reactions. Two different containers are used and a wire is used to drive the electrons from one side to the other and a Voltaic/Galvanic cell is created. It is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A salt bridge also connects to the half-cells. The reading of the voltmeter gives the cell voltage or cell potential or electromotive force. If \[\ce{E^0_{cell}}\] is positive the reaction is spontaneous and if it is negative the reaction is non-spontaneous and is referred to as electrolytic cell. Electrolysis refers to the decomposition of a substance by an electric current. One mole of electric charge when passed through a cell will discharge half a mole of a divalent metal ion such as Cu2+. This was first formulated by Faraday in the form of laws of electrolysis.
|
- Is silver plate the anode or cathode? (1)
- What will happen if the salt bridge is removed? (1)
- When does electrochemical cell behaves like an electrolytic cell? (1)
- (i) What will happen to the concentration of Zn2+ and Ag+ when Ecell = 0. (1)
(ii) Why does conductivity of a solution decreases with dilution? (1)
OR
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2mol-1. Calculate the conductivity of this solution. (2)
State the term for the following:
Two metal plates or wires through which the current enters and leaves the electrolytic cell.