Advertisements
Advertisements
प्रश्न
The molar conductivity of CH3COOH at infinite dilution is 390 Scm2/mol. Using the graph and given information, the molar conductivity of CH3COOK will be:
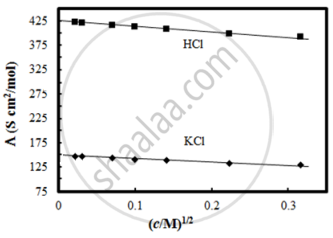
विकल्प
100 Scm2/mol
115 Scm2/mol
150 Scm2/mol
125 Scm2/mol
उत्तर
115 Scm2/mol
Explanation:
Λ°CH3COOK = Λ°CH3COOH + Λ°KCl – Λ°HCl
= 390 + 150 – 425
= 115 Scm2/mol
APPEARS IN
संबंधित प्रश्न
The conductivity of 0.02M AgNO3 at 25°C is 2.428 x 10-3 Ω-1 cm-1. What is its molar
conductivity?
Molar conductivity denoted by the symbol Λm is related to the conductivity of the solution by the equation (k is the conductivity and c is the concentration).
Kohlrausch law of independent migration of ions states ____________.
When acidulated water (dil.H2SO4 solution) is electrolysed, will the pH of the solution be affected? Justify your answer.
Match the items of Column I and Column II on the basis of data given below:
`E_("F"_2//"F"^-)^Θ` = 2.87 V, `"E"_(("Li"^(+))//("Li"^-))^Θ` = − 3.5V, `"E"_(("Au"^(3+))//("Au"))^Θ` = 1.4 V, `"E"_(("Br"_(2))//("Br"^-))^Θ` = 1.09 V
| Column I | Column II |
| (i) F2 | (a) metal is the strongest reducing agent |
| (ii) Li | (b) metal ion which is the weakest oxidising agent |
| (iii) Au3+ | (c) non metal which is the best oxidising agent |
| (iv) Br– | (d) unreactive metal |
| (v) Au | (e) anion that can be oxidised by Au3+ |
| (vi) Li+ | (f) anion which is the weakest reducing agent |
| (vii) F– | (g) metal ion which is an oxidising agent |
Assertion: `"E"_("Ag"^+ //"Ag")` increases with increase in concentration of Ag+ ions.
Reason: `"E"_("Ag"^+ //"Ag")` has a positive value.
An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to :-
The molar conductance of NaCl, HCl, and CH3COONa at infinite dilution are 126.45, 426.16, and 91.0 S cm2 mol−1 respectively. The molar conductance of CH3COOH at infinite dilution is. Choose the right option for your answer.
Given below are two statements:
Statements I: The limiting molar conductivity of KCl (strong electrolyte) is higher compared to that of CH3COOH (weak electrolyte).
Statement II: Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
| Rahul set up an experiment to find the resistance of aqueous KCl solution for different concentrations at 298 K using a conductivity cell connected to a Wheatstone bridge. He fed the Wheatstone bridge with a.c. power in the audio frequency range 550 to 5000 cycles per second. Once the resistance was calculated from the null point, he also calculated the conductivity K and molar conductivity ∧m and recorded his readings in tabular form. |
| S. No. | Conc. (M) |
k S cm−1 | ∧m S cm2 mol−1 |
| 1. | 1.00 | 111.3 × 10−3 | 111.3 |
| 2. | 0.10 | 12.9 × 10−3 | 129.0 |
| 3. | 0.01 | 1.41 × 10−3 | 141.0 |
Answer the following questions:
(a) Why does conductivity decrease with dilution? (1)
(b) If `∧_"m"^0` of KCl is 150.0 S cm2 mol−1, calculate the degree of dissociation of 0.01 M KCI. (1)
(c) If Rahul had used HCl instead of KCl then would you expect the ∧m values to be more or less than those per KCl for a given concentration? Justify. (2)
OR
(c) Amit a classmate of Rahul repeated the same experiment with CH3COOH solution instead of KCl solution. Give one point that would be similar and one that would be different in his observations as compared to Rahul. (2)
