Advertisements
Advertisements
प्रश्न
Assertion: `"E"_("Ag"^+ //"Ag")` increases with increase in concentration of Ag+ ions.
Reason: `"E"_("Ag"^+ //"Ag")` has a positive value.
विकल्प
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
उत्तर
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Explanation:
\[\ce{Ag- + e- -> Ag}\]
`"E"_("Ag"^+//"Ag") = "E"_("Ag"^+//"Ag")^0 - 0.059/1 log 1/(["Ag"^+])`
= `"E"_("Ag"^+//"Ag")^0 + 0.059["Ag"^+]`
On increasing \[\ce{[Ag+], E_{Ag^+/Ag}}\] will increase and it has a positive value.
APPEARS IN
संबंधित प्रश्न
The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm−1. Calculate its molar conductivity.
State Kohlrausch law of independent migration of ions.
Why does the conductivity of a solution decrease with dilution?
The molar conductivity of 0.025 mol L−1 methanoic acid is 46.1 S cm2 mol−1. Calculate its degree of dissociation and dissociation constant. Given \[\ce{λ^0_{(H^+)}}\] = 349.6 S cm2 mol−1 and \[\ce{λ^0_{(HCOO^-)}}\] = 54.6 S cm2 mol−1.
How can you determine limiting molar conductivity, 0 m for strong electrolyte and weak electrolyte?
The S.I. unit of cell constant for conductivity cell is __________.
\[\ce{Λ^0_m}_{(NH_4OH)}\] is equal to ______.
Consider figure and answer the question to given below.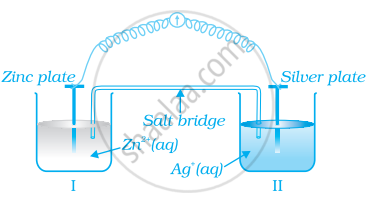
How will the concentration of Zn2+ ions and Ag+ ions be affected after the cell becomes ‘dead’?
The specific conductance of 2.5 × 10-4 M formic acid is 5.25 × 10-5 ohm-1 cm-1. Calculate its molar conductivity and degree of dissociation.
Given `λ°_("H"^+)` = 349.5 ohm-1 cm2 mol-1 and
`λ°_("HCOO"^-) = 50.5 " ohm"^-1 "cm"^2 "mol"^-1`
The solution of two electrolytes A and B are diluted. ^m of B increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Give a reason.
