Advertisements
Advertisements
प्रश्न
\[\ce{Λ^0_m}_{(NH_4OH)}\] is equal to ______.
विकल्प
\[\ce{Λ^0_m}_{(NH_4OH)} + \ce{Λ^0_m_{(NH_4Cl) } - \ce{Λ^0}_{(HCl)}}\]
\[\ce{Λ^0_m}_{(NH_4Cl)} + \ce{Λ^0_m_{(NaOH) } - \ce{Λ^0}_{(NaCl)}}\]
\[\ce{Λ^0_m}_{(NH_4Cl)} + \ce{Λ^0_m_{(NaCl) } - \ce{Λ^0}_{NaOH)}}\]
\[\ce{Λ^0_m}_{(NaOH)} + \ce{Λ^0_m_{(NACl) } - \ce{Λ^0}_{(NH_4Cl)}}\]
उत्तर
\[\ce{Λ^0_m}_{(NH_4Cl)} + \ce{Λ^0_m_{(NaOH) } - \ce{Λ^0}_{(NaCl)}}\]
Explanation:
(i) \[\ce{NH4CI ⇌ NH^{+}4 + Cl-}\]
(ii) \[\ce{NaCI ⇌ Na+ + Cl-}\]
(iii) \[\ce{NaOH ⇌ Na+ + OH-}\]
(iv) \[\ce{NH4OH ⇌ NH^{+}4 + OH-}\]
To get equation (iv),
\[\ce{Λ^0_m}_{(NH_4Cl)} + \ce{Λ^0_m_{(NaOH) } - \ce{Λ^0}_{(NaCl)} = \ce{Λ^0_m}_{(NH_4OH)}}\]
APPEARS IN
संबंधित प्रश्न
The molar conductivity of cation and anion of salt BA are 180 and 220 mhos respectively. The molar conductivity of salt BA at infinite dilution is_____________ .
(a) 90 mhos.cm2
(b) 110 mhos.cm2.mol-1
(c) 200 mhos.cm2.mol-1
(d) 400 mhos.cm2.mol-1
State Kohlrausch Law
State Kohlrausch law of independent migration of ions.
Why does the conductivity of a solution decrease with dilution?
Calculate the degree of dissociation (α) of acetic acid if its molar conductivity (Λm) is 39.05 S cm2 mol−1.
Given λ°(H+) = 349.6 S cm2 mol−1 and λ°(CH3COO−) = 40.9 S cm2 mol−1
The S.I. unit of cell constant for conductivity cell is __________.
Match the items of Column I and Column II on the basis of data given below:
`E_("F"_2//"F"^-)^Θ` = 2.87 V, `"E"_(("Li"^(+))//("Li"^-))^Θ` = − 3.5V, `"E"_(("Au"^(3+))//("Au"))^Θ` = 1.4 V, `"E"_(("Br"_(2))//("Br"^-))^Θ` = 1.09 V
| Column I | Column II |
| (i) F2 | (a) metal is the strongest reducing agent |
| (ii) Li | (b) metal ion which is the weakest oxidising agent |
| (iii) Au3+ | (c) non metal which is the best oxidising agent |
| (iv) Br– | (d) unreactive metal |
| (v) Au | (e) anion that can be oxidised by Au3+ |
| (vi) Li+ | (f) anion which is the weakest reducing agent |
| (vii) F– | (g) metal ion which is an oxidising agent |
Consider figure and answer the question to given below.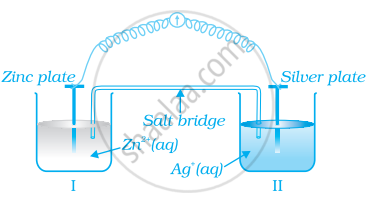
How will the concentration of Zn2+ ions and Ag+ ions be affected after the cell becomes ‘dead’?
Which of the following halogen acids is the strongest reducing agent?
Which of the following solutions will have the highest conductivity at 298 K?
