Advertisements
Advertisements
प्रश्न
State Kohlrausch Law
उत्तर १
Kohlrausch Law:
The equivalent conductivity of an electrolyte at infinite dilution is the sum of two values on depending upon the cation and the other upon the anion.
उत्तर २
Kohlrausch's law states that at infinite dilution of the solution, each ion of electrolyte migrates independently of its co-ions and contribute independently to the total molar conductivity irrespective of the nature of other ion.
APPEARS IN
संबंधित प्रश्न
Resistance of conductivity cell filled with 0.1 M KCl solution is 100 ohms. If the resistance of the same cell when filled with 0.02 M KCl solution is 520 ohms, calculate the conductivity and molar conductivity of 0.02 M KCl solution. [Given: Conductivity of 0.1 M KCl solution is 1.29 S m-1 .]
State Kohlrausch’s law of independent migration of ions.
The molar conductivity of cation and anion of salt BA are 180 and 220 mhos respectively. The molar conductivity of salt BA at infinite dilution is_____________ .
(a) 90 mhos.cm2
(b) 110 mhos.cm2.mol-1
(c) 200 mhos.cm2.mol-1
(d) 400 mhos.cm2.mol-1
The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm−1. Calculate its molar conductivity.
State Kohlrausch law of independent migration of ions.
The molar conductivity of 0.025 mol L−1 methanoic acid is 46.1 S cm2 mol−1. Calculate its degree of dissociation and dissociation constant. Given \[\ce{λ^0_{(H^+)}}\] = 349.6 S cm2 mol−1 and \[\ce{λ^0_{(HCOO^-)}}\] = 54.6 S cm2 mol−1.
10.0 grams of caustic soda when dissolved in 250 cm3 of water, the resultant gram molarity of solution is _______.
(A) 0.25 M
(B) 0.5 M
(C) 1.0 M
(D) 0.1 M
Write mathematical expression of molar conductivity of the given solution at infinite dilution.
Define the following terms :
Limiting molar conductivity
How can you determine limiting molar conductivity, 0 m for strong electrolyte and weak electrolyte?
The S.I. unit of cell constant for conductivity cell is __________.
Molar conductivity denoted by the symbol Λm is related to the conductivity of the solution by the equation (k is the conductivity and c is the concentration).
\[\ce{Λ^0_m}_{(NH_4OH)}\] is equal to ______.
Solutions of two electrolytes ‘A’ and ‘B’ are diluted. The Λm of ‘B’ increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Justify your answer.
When acidulated water (dil.H2SO4 solution) is electrolysed, will the pH of the solution be affected? Justify your answer.
Write the cell reaction of a lead storage battery when it is discharged. How does the density of the electrolyte change when the battery is discharged?
Assertion: `"E"_("Ag"^+ //"Ag")` increases with increase in concentration of Ag+ ions.
Reason: `"E"_("Ag"^+ //"Ag")` has a positive value.
Assertion: Copper sulphate can be stored in zinc vessel.
Reason: Zinc is less reactive than copper.
Consider figure and answer the question to given below.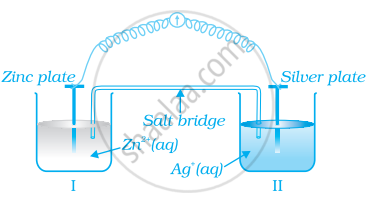
How will the concentration of Zn2+ ions and Ag+ ions be affected after the cell becomes ‘dead’?
Which of the following increases with the increase in the concentration of the solution?
The molar conductance of \[\ce{NaCl, HCl}\] and \[\ce{CH3COONa}\] at infinite dilution are 126.45, 426.16 and 91.0 S cm2 mol−1 respectively. The molar conductance of \[\ce{CH3COOH}\] at infinite dilution is. Choose the right option for your answer.
Molar conductivity of substance “A” is 5.9 × 103 S/m and “B” is 1 × 10–16 S/m. Which of the two is most likely to be copper metal and why?
Given below are two statements:
Statements I: The limiting molar conductivity of KCl (strong electrolyte) is higher compared to that of CH3COOH (weak electrolyte).
Statement II: Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
| Rahul set up an experiment to find the resistance of aqueous KCl solution for different concentrations at 298 K using a conductivity cell connected to a Wheatstone bridge. He fed the Wheatstone bridge with a.c. power in the audio frequency range 550 to 5000 cycles per second. Once the resistance was calculated from the null point, he also calculated the conductivity K and molar conductivity ∧m and recorded his readings in tabular form. |
| S. No. | Conc. (M) |
k S cm−1 | ∧m S cm2 mol−1 |
| 1. | 1.00 | 111.3 × 10−3 | 111.3 |
| 2. | 0.10 | 12.9 × 10−3 | 129.0 |
| 3. | 0.01 | 1.41 × 10−3 | 141.0 |
Answer the following questions:
(a) Why does conductivity decrease with dilution? (1)
(b) If `∧_"m"^0` of KCl is 150.0 S cm2 mol−1, calculate the degree of dissociation of 0.01 M KCI. (1)
(c) If Rahul had used HCl instead of KCl then would you expect the ∧m values to be more or less than those per KCl for a given concentration? Justify. (2)
OR
(c) Amit a classmate of Rahul repeated the same experiment with CH3COOH solution instead of KCl solution. Give one point that would be similar and one that would be different in his observations as compared to Rahul. (2)
The unit of molar conductivity is ______.
The specific conductance of 2.5 × 10-4 M formic acid is 5.25 × 10-5 ohm-1 cm-1. Calculate its molar conductivity and degree of dissociation.
Given `λ°_("H"^+)` = 349.5 ohm-1 cm2 mol-1 and
`λ°_("HCOO"^-) = 50.5 " ohm"^-1 "cm"^2 "mol"^-1`
Suggest a way to determine the `∧_"m"^∘`value of water.
