Advertisements
Advertisements
प्रश्न
The unit of molar conductivity is ______.
विकल्प
S cm−2 mol−1
S cm2 mol−1
S−1 cm2 mo1−1
S cm2 mol
उत्तर
The unit of molar conductivity is S cm2 mol−1.
APPEARS IN
संबंधित प्रश्न
Define the following terms: Molar conductivity (⋀m)
Conductivity of 0.00241 M acetic acid is 7.896 × 10−5 S cm−1. Calculate its molar conductivity and if `∧_"m"^0` for acetic acid is 390.5 S cm2 mol−1, what is its dissociation constant?
Calculate the degree of dissociation (α) of acetic acid if its molar conductivity (Λm) is 39.05 S cm2 mol−1.
Given λ°(H+) = 349.6 S cm2 mol−1 and λ°(CH3COO−) = 40.9 S cm2 mol−1
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2) following curves are obtained for two electrolytes A and B : 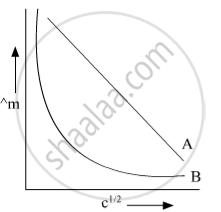
Answer the following:
(i) predict the nature of electrolytes A and B.
(ii) What happens on the extrapolation of ∧m to concentration approaching for electrolytes A and B?
Which of the statements about solutions of electrolytes is not correct?
\[\ce{Λ^0_m}_{(NH_4OH)}\] is equal to ______.
The limiting molar conductivities for Nacl, KBr and KCI are 126, 152 and 150 S cm2 mol–1 respectively. The limiting molar conductivity for Na Br is:-
The molar conductivity of 0.007 M acetic acid is 20 S cm2 mol−1. What is the dissociation constant of acetic acid? Choose the correct option.
`[(Λ_("H"^+)^ο = 350 "S" "cm"^2 "mol"^-1), (Λ_("CH"_3"COO"^-)^ο = 50 "S" "cm"^2 "mol"^-1)]`
Which of the following solutions of KCl will have the highest value of molar conductivity?
Conductivity of 2 × 10−3 M methanoic acid is 8 × 10−5 S cm−1. Calculate its molar conductivity and degree of dissociation if `∧_"m"^0` for methanoic acid, is 404 S cm2 mol−3.
