Advertisements
Advertisements
प्रश्न
When acidulated water (dil.H2SO4 solution) is electrolysed, will the pH of the solution be affected? Justify your answer.
उत्तर
Anode: \[\ce{2H2O(l) -> O2(g) + 4H + (aq) + 4e^{-}}\]
Cathode: \[\ce{4H^{+} + 4e^{-} -> 2H2}\]
Overall cell reaction: \[\ce{2H2O(l) -> O2(g) + 2H2(g)}\]
pH remains the same because concentration of H+ ions remains constant.
APPEARS IN
संबंधित प्रश्न
The conductivity of 0.001 mol L-1 solution of CH3COOH is 3.905× 10-5 S cm-1. Calculate its molar conductivity and degree of dissociation (α) Given λ°(H+)= 349.6 S cm2 mol-1 and λ°(CH3COO)= 40.9S cm2mol-1.
Why conductivity of an electrolyte solution decreases with the decrease in concentration ?
Define the following terms :
Limiting molar conductivity
A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4and ZnSO4 until 2.8g of Fe deposited at the cathode of cell X. How long did the current flow? Calculate the mass of Zn deposited at the cathode of cell Y.
(Molar mass: Fe=56g mol-1,Zn=65.3g mol-1,1F=96500C mol-1)
Molar conductivity of ionic solution depends on:
(i) temperature.
(ii) distance between electrodes.
(iii) concentration of electrolytes in solution.
(iv) surface area of electrodes.
Assertion: `"E"_("Ag"^+ //"Ag")` increases with increase in concentration of Ag+ ions.
Reason: `"E"_("Ag"^+ //"Ag")` has a positive value.
Solutions of two electrolytes ‘A’ and ‘B’ are diluted. The Λm of ‘B’ increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Justify your answer. Graphically show the behavior of ‘A’ and ‘B’.
An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to :-
The variation of molar conductivity with concentration of an electrolyte (X) m aqueous solution is shown in the given figure.
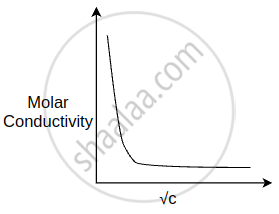
The electrolyte X is ______.
Which of the following solutions will have the highest conductivity at 298 K?
