Advertisements
Advertisements
Question
The specific conductance of 2.5 × 10-4 M formic acid is 5.25 × 10-5 ohm-1 cm-1. Calculate its molar conductivity and degree of dissociation.
Given `λ°_("H"^+)` = 349.5 ohm-1 cm2 mol-1 and
`λ°_("HCOO"^-) = 50.5 " ohm"^-1 "cm"^2 "mol"^-1`
Solution
Given: K = 5.25 × 10-5 ohm-1 cm-1
C = 2.5 × 10-4 M
Formula used `lambda_m = (1000 xx "K")/"C", alpha = lambda_m/lambda_0`
Molar conductivity `(lambda_m) = (1000 xx "K")/"C"`
`(lambda_m) = (100 xx 5.25 xx 10^-5)/(2.5 xx 10^-4)`
= 210 s cm2 mol-1
\[\ce{\lambda^\circ_{{\phantom{..}}CH_3COOH} = (\lambda^\circ_{\phantom{..}H} + \lambda^\circ_{\phantom{..}HCOO^-})}\]
= (349.5 + 50.5)
Degree of dissociation:
`alpha = lambda_m/lambda_0 = 210/400` = 0.525
α = 52.5%
APPEARS IN
RELATED QUESTIONS
The conductivity of 0.001 mol L-1 solution of CH3COOH is 3.905× 10-5 S cm-1. Calculate its molar conductivity and degree of dissociation (α) Given λ°(H+)= 349.6 S cm2 mol-1 and λ°(CH3COO)= 40.9S cm2mol-1.
State Kohlrausch law of independent migration of ions.
Define the following terms :
Limiting molar conductivity
How can you determine limiting molar conductivity, 0 m for strong electrolyte and weak electrolyte?
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2), following curves are obtained for two electrolytes A and B:
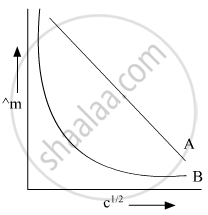
Answer the following:
(i) Predict the nature of electrolytes A and B.
(ii) What happens on extrapolation of ∧m to concentration approaching zero for electrolytes A and B?
Molar conductivity denoted by the symbol Λm is related to the conductivity of the solution by the equation (k is the conductivity and c is the concentration).
Kohlrausch law of independent migration of ions states ____________.
The molar conductance of NaCl, HCl, and CH3COONa at infinite dilution are 126.45, 426.16, and 91.0 S cm2 mol−1 respectively. The molar conductance of CH3COOH at infinite dilution is. Choose the right option for your answer.
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
| Rahul set up an experiment to find the resistance of aqueous KCl solution for different concentrations at 298 K using a conductivity cell connected to a Wheatstone bridge. He fed the Wheatstone bridge with a.c. power in the audio frequency range 550 to 5000 cycles per second. Once the resistance was calculated from the null point, he also calculated the conductivity K and molar conductivity ∧m and recorded his readings in tabular form. |
| S. No. | Conc. (M) |
k S cm−1 | ∧m S cm2 mol−1 |
| 1. | 1.00 | 111.3 × 10−3 | 111.3 |
| 2. | 0.10 | 12.9 × 10−3 | 129.0 |
| 3. | 0.01 | 1.41 × 10−3 | 141.0 |
Answer the following questions:
(a) Why does conductivity decrease with dilution? (1)
(b) If `∧_"m"^0` of KCl is 150.0 S cm2 mol−1, calculate the degree of dissociation of 0.01 M KCI. (1)
(c) If Rahul had used HCl instead of KCl then would you expect the ∧m values to be more or less than those per KCl for a given concentration? Justify. (2)
OR
(c) Amit a classmate of Rahul repeated the same experiment with CH3COOH solution instead of KCl solution. Give one point that would be similar and one that would be different in his observations as compared to Rahul. (2)
Discuss the variation of conductivity and molar conductivity with concentration.
