HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2012-2013
Date: March 2013
Advertisements
In body centred cubic structure the space occupied is about
(A) 68% (B) 53%
(C) 38% (D) 32%
Chapter: [0.01] Solid State
For a gaseous reaction the unit of rate of reaction is
(A) L atm s-1
(B) atm mol-1 s-1
(C) atm s1
(D) mol s
Chapter:
Which of the following compounds contain S = O as well as S = S bonds?
(A) Sulphuric acid
(B) Thiosulphuric acid
(C) Sulphurous acid
(D) Thiosulphurous acid
Chapter: [7.02] Group 16 Elements
Which of the following solutions shows maximum depression in freezing point?
(A) 0.5 M Li2SQ4
(B) 1 M NaCl
(C) 0.5 M A12(SO4)3
(D) 0.5 MBaC12
Chapter: [0.02] Solutions and Colligative Properties
For a chemical reaction dS=0.035 kJ/k and dH=20kJ. At what temperature does the reaction turn nonspontaneous?
5.14 K
57.14 K
571.4 K
5714.0 K
Chapter: [0.03] Chemical Thermodynamics and Energetic
The standard e.m.f of the following cell is 0.463 V
`Cu|Cu_(1m)^(++)`
What is the standard potential of Cu electrode?
(A) 1.137 V
(B) 0.337 V
(C) 0.463 V
(D) - 0.463 V
Chapter: [0.04] Electrochemistry
Fe2O3 is reduced to spongy iron near the top of blast furance by
(A) H2
(B) CaO
(C) SiO2
(D) CO
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Distinguish between crystalline solid and amorphous solid
Chapter: [0.01] Solid State
Write mathematical expression of molar conductivity of the given solution at infinite dilution.
Chapter: [0.04] Electrochemistry
Write cell reaction in lead storage battery during discharge.
Chapter: [0.04] Electrochemistry
Draw structure and write geometry of PCl3 and PCl5.
Chapter: [7.01] Group 15 Elements
Prove that ΔH=ΔU+ΔnRT. what is the condition under which ΔU=ΔH?
Chapter: [0.03] Chemical Thermodynamics and Energetic
Mention names and formulae of two ores of alumunium.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Derive the relationship between relative lowering of vapour pressure and molar mass of nonvolatile solute.
Chapter: [0.02] Solutions and Colligative Properties
What is pseudo first order reaction? Give one· example of it.
Chapter: [0.05] Chemical Kinetics
Calculate the mole fraction and molality of HNO3 in solution contaning 12.2%HNO3 (Given atomic mases:H=1, N=13,O=16)
Chapter: [0.02] Solutions and Colligative Properties
Advertisements
Consider the reaction
`3I_((aq))^-) +S_2O_8^(2-)->I_(3(aq))^-) + 2S_2O_4^(2-)`
At particular time t, `(d[SO_4^(2-)])/dt=2.2xx10^(-2)"M/s"`
What are the values of the following at the same time?
a. `-(d[I^-])/dt`
b. `-(d[S_2O_8^(2-)])/dt`
c. `-(d[I_3^-])/dt`
Chapter: [0.05] Chemical Kinetics
300 M mol of perfect gas occupies 13 L at 320 K. Calculate the work done in joules when the gas expands-
(a) isothermally against a Constant external pressure of 0.20atm.
(b) isothermal and reversible process.
(c) into vaccum until the volume of gas is increased by 3L (R=8.314J mol-1 K-1)
Chapter: [0.03] Chemical Thermodynamics and Energetic
What is the action of Excess of air on ammonia ?
Chapter: [7.01] Group 15 Elements
What is the action of Excess of chlorine on ammonia?
Chapter: [7.01] Group 15 Elements
What is the action of Na Metal on ammonia?
Chapter: [7.01] Group 15 Elements
Explain with reason sign conventions of ΔS in the following reaction
N2(g) + 3H2(g) → 2NH3(g)
Chapter: [0.01] Solid State
Explain with reason sign conventions of ΔS in the following reaction
CO2(g) → CO2(g)
Chapter: [0.01] Solid State
Explain the following term
Smelting
Chapter: [0.08] Transition and Inner Transition Elements
Explain the following term
Flux
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Gold occurs as face centred cube and has a density of 19.30 kg dm-3. Calculate atomic radius of gold (Molar mass of Au = 197)
Chapter: [0.01] Solid State
a. Explain the trends in the following properties with reference to group 16:
1 Atomic radii and ionic radii
2 Density
3 ionisation enthalpy
4 Electronegativity
b. In the electolysis of AgNO3 solution 0.7g of Ag is deposited after a certain period of time. Calulate the quantity of electricity required in coulomb. (Molar mass of Ag is 107.9g mol-1)
Chapter: [7.02] Group 16 Elements
Explain the term osmosis.
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
ln which pair highest oxidation states of transition metals are found:
nitriles and chlorides
fluorides and chlorides
fluorides and oxides
nitriles and oxides
Chapter: [8.01] D-block Elements
which of the following carbocations is least stable?
(A) 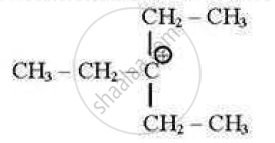
(B)![]()
(C)![]()
(D)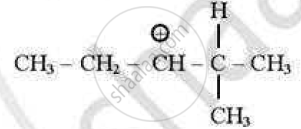
Chapter: [10.01] Haloalkanes [10.02] Haloarenes
Compound having general formula 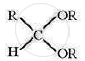 is called
is called
(A) diester
(B) acid anhydride
(C) hemiacetal
(D) acetal
Chapter: [12.01] Aldehydes and Ketones
The complex ion `[Co(H_2O)_5 (ONO)]^(2+) `
(A) linkage isomer
(B) ionisation isomer
(C) co-ordination isomer
(D) geometrical isomer
Chapter: [0.09] Coordination Compounds
Inflammation of the tongue is due to the deficiency of:
Vitamin B1
Vitamin B2
Vitamin B5
Vitamin B6
Chapter: [14.03] Vitamins
Advertisements
Identify the compound 'B' in the following series of reaction:
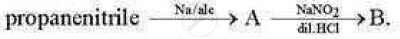
(A) n-propyl chloride
(B) Propanamine
(C) n-propyl alcohol
(D) Isoproply alcohol
Chapter: [11.01] Alcohols
Which of the following reagents is best for the following conversion?
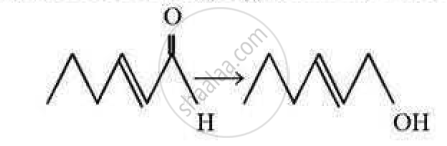
(A) LiAlH4
(B) ![]()
(C) H2/Ni, 453 K
(D) Zn-Hg+HCl(con)
Chapter: [11.01] Alcohols
Calculate magnetic moment of `Fe_((aq))^(2+) ion (Z=26).`
Chapter: [8.01] D-block Elements
How is ethanol prepared from methanal by using Grignard reagent?
Chapter: [11.01] Alcohols
Write the chemical reaction to prepare novolac polymer.
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Why does p-nitrochlorobenzene undergo displacement reactions readily with attack of nucleophilic HOΘ ion?
Chapter: [10.02] Haloarenes
What is the action of bromine in alkaline medium on
i. CH3CH2NO2
ii. 
Chapter: [10.01] Haloalkanes
How is 4-methylpent-3-en-2-one obtained from propan-2-one?
Chapter: [12.01] Aldehydes and Ketones
Write the structure of simple triglycerides.
Chapter: [14.02] Proteins
Write the different oxidation states of manganese.
Chapter: [8.01] D-block Elements
Why +2 oxidation state of manganese is more stable?
Chapter: [8.01] D-block Elements
How are the following compounds prepared?
benzaldehyde from benzene
Chapter: [12.01] Aldehydes and Ketones
How are the following compounds prepared?
acetophenone from benzene
Chapter: [12.01] Aldehydes and Ketones
How are the following compounds prepared?
benzaldehyde from benzoyl chloride
Chapter: [12.01] Aldehydes and Ketones
Write the structure of a nucleotide
Chapter: [14.04] Nucleic Acids
Write the structure of a nucleoside
Chapter: [14.04] Nucleic Acids
Write the fomulae of the following compounds
Sodium hexanitrito- N - cobaltate (III)
Chapter: [0.09] Coordination Compounds
Write the fomulae of the following compounds :
Tetraaquodichlorochromium (III) chloride
Chapter: [0.09] Coordination Compounds
Write the fomulae of the following compounds
Potassium tetracyanoaurate (III) ion
Chapter: [0.09] Coordination Compounds
Explain the following term: Homopolymers
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Explain the mechanism of cleansing action of soaps.
Chapter: [16.03] Cleansing Agents
Write balanced chemical equations for the action of phosphorous trichloride on propan-2-ol
Chapter:
Write balanced chemical equations for the action of hydrogen bromide on styrene in the presence of a peroxide
Chapter: [16.03] Cleansing Agents
Write balanced chemical equations for the action of methyl bromide on silver propanoate
Chapter: [16.03] Cleansing Agents
Write a short note on Hoffmann bromamide degradation.
Chapter: [0.13] Amines [13.01] Amines
Explain the mechanism of action of hydroiodic acid on 3-methylbutan-2-ol.
Chapter: [0.13] Amines [13.01] Amines
Mention 'two' uses of propan-2-one.
Chapter: [0.13] Amines [13.01] Amines
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2012 - 2013
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2013 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
