Advertisements
Advertisements
Question
Consider the reaction
`3I_((aq))^-) +S_2O_8^(2-)->I_(3(aq))^-) + 2S_2O_4^(2-)`
At particular time t, `(d[SO_4^(2-)])/dt=2.2xx10^(-2)"M/s"`
What are the values of the following at the same time?
a. `-(d[I^-])/dt`
b. `-(d[S_2O_8^(2-)])/dt`
c. `-(d[I_3^-])/dt`
Solution
Rate of reaction
`-(1/3)(d[I^-])/dt = -(d[S_2O_8^-2])/dt = +(d[I_3^-])/dt = +(1/2)(d[SO_4^-2])/dt`
`(d[SO_4^-2])/dt=2.2xx10^-2 "M/s"`
(a)
`-(1/3)(d[I^-])/dt = (1/2)(d[SO_4^-2])/dt`
`-(d[I^-])/dt=3/2xx2.2xx10^-2`
`-(d[I^-])/dt=3.3xx10^-2 " M/s"`
(b)
`-(d[S_2O_s^(-2)])/dt=(1/2)(d[SO_4^-2])/dt`
`-(d[S_2O_s^(-2)])/dt=1/2xx2.2xx10^-2`
`-(d[S_2O_s^(-2)])/dt=1.1xx10^-2 " M/s"`
(c)
`(d[I_3^-])/dt=(1/2)(d[SO_4^-2])/dt`
`(d[I_3^-])/dt=1/2xx2.2xx10^-2`
`(d[I_3^-])/dt=1.1xx10^-2`
`-(d[I_3^-])/dt=-1.1xx10^-2 " M/s"`
APPEARS IN
RELATED QUESTIONS
Explain a graphical method to determine activation energy of a reaction.
(b) Rate constant ‘k’ of a reaction varies with temperature ‘T’ according to the equation:
`logk=logA-E_a/2.303R(1/T)`
Where Ea is the activation energy. When a graph is plotted for `logk Vs. 1/T` a straight line with a slope of −4250 K is obtained. Calculate ‘Ea’ for the reaction.(R = 8.314 JK−1 mol−1)
The rate constant of a first order reaction increases from 4 × 10−2 to 8 × 10−2 when the temperature changes from 27°C to 37°C. Calculate the energy of activation (Ea). (log 2 = 0.301, log 3 = 0.4771, log 4 = 0.6021)
The rate constant of a first order reaction increases from 2 × 10−2 to 4 × 10−2 when the temperature changes from 300 K to 310 K. Calculate the energy of activation (Ea).
(log 2 = 0.301, log 3 = 0.4771, log 4 = 0.6021)
The rate constant for the decomposition of N2O5 at various temperatures is given below:
| T/°C | 0 | 20 | 40 | 60 | 80 |
| 105 × k/s−1 | 0.0787 | 1.70 | 25.7 | 178 | 2140 |
Draw a graph between ln k and `1/"T"` and calculate the values of A and Ea. Predict the rate constant at 30º and 50ºC.
The rate constant for the decomposition of hydrocarbons is 2.418 × 10−5 s−1 at 546 K. If the energy of activation is 179.9 kJ/mol, what will be the value of pre-exponential factor?
The decomposition of A into product has value of k as 4.5 × 103 s−1 at 10°C and energy of activation 60 kJ mol−1. At what temperature would k be 1.5 × 104 s−1?
In the Arrhenius equation for a first order reaction, the values of ‘A’ of ‘Ea’ are 4 x 1013 sec-1 and 98.6 kJ mol-1 respectively. At what temperature will its half life period be 10 minutes?
[R = 8.314 J K-1 mol-1]
Define activation energy.
What is the effect of adding a catalyst on Activation energy (Ea)
A first-order reaction is 50% completed in 40 minutes at 300 K and in 20 minutes at 320 K. Calculate the activation energy of the reaction. (Given : log 2 = 0·3010, log 4 = 0·6021, R = 8·314 JK–1 mol–1)
Explain the following terms :
Half life period of a reaction (t1/2)
Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction. (Given : log2 = 0.3010,log 3 = 0.4771, log5 = 0.6990).
Predict the main product of the following reactions: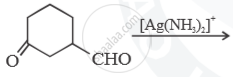
The chemical reaction in which reactants require high amount of activation energy are generally ____________.
The rate of chemical reaction becomes double for every 10° rise in temperature because of ____________.
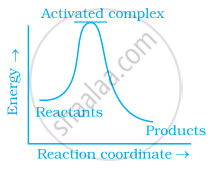
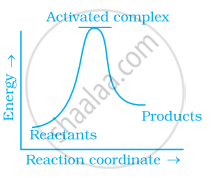
Which of the following graphs represents exothermic reaction?
(a)
(b)
(c)
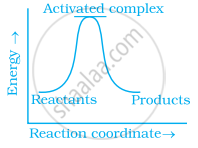
During decomposition of an activated complex:
(i) energy is always released
(ii) energy is always absorbed
(iii) energy does not change
(iv) reactants may be formed
Mark the incorrect statements:
(i) Catalyst provides an alternative pathway to reaction mechanism.
(ii) Catalyst raises the activation energy.
(iii) Catalyst lowers the activation energy.
(iv) Catalyst alters enthalpy change of the reaction.
Oxygen is available in plenty in air yet fuels do not burn by themselves at room temperature. Explain.
The slope of Arrhenius Plot `("In" "k" "v"//"s" 1/"T")` of first-order reaction is −5 × 103 K. The value of Ea of the reaction is. Choose the correct option for your answer. [Given R = 8.314 JK−1mol−1]
The equation k = `(6.5 xx 10^12 "s"^(-1))"e"^(- 26000 " K"//"T")` is followed for the decomposition of compound A. The activation energy for the reaction is ______ kJ mol-1. (Nearest integer) (Given: R = 8.314 JK-1 mol-1)
The decomposition of N2O into N2 and O2 in the presence of gaseous argon follows second-order kinetics, with k = (5.0 × 1011 L mol−1 s−1) `"e"^(-(29000 "K")/"T")`. Arrhenius parameters are ______ kJ mol−1.
An exothermic reaction X → Y has an activation energy 30 kJ mol-1. If energy change ΔE during the reaction is - 20 kJ, then the activation energy for the reverse reaction in kJ is ______.
A first-order reaction is 50% complete in 30 minutes at 300 K and in 10 minutes at 320 K. Calculate activation energy (Ea) for the reaction. [R = 8.314 J K−1 mol−1]
[Given: log 2 = 0.3010, log 3 = 0.4771, log 4 = 0.6021]
