Advertisements
Advertisements
Question
Which of the following graphs represents exothermic reaction?
(a)
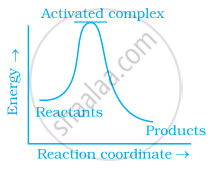
(b)
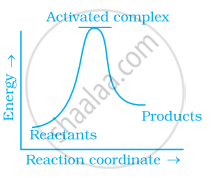
(c)
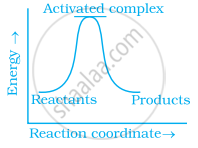
Options
(a) only
(b) only
(c) only
(a) and (b)
Solution
(a) only
Explanation:
Exothermic reaction is a chemical reaction in which the enthalpy change is negative and energy is released in the form of light and heat.
As we all know, ΔH is negative.
Activation energy (forward reaction) – (backward reaction)
(Forward reaction) – Ea (backward reaction)
The product's exothermic energy exceeds the activation energy of the reactant.
APPEARS IN
RELATED QUESTIONS
What is the effect of adding a catalyst on Activation energy (Ea)
The rate of chemical reaction becomes double for every 10° rise in temperature because of ____________.
Activation energy of a chemical reaction can be determined by ______.
Why in the redox titration of \[\ce{KMnO4}\] vs oxalic acid, we heat oxalic acid solution before starting the titration?
What happens to most probable kinetic energy and the energy of activation with increase in temperature?
In respect of the eqn k = \[\ce{Ae^{{-E_a}/{RT}}}\] in chemical kinetics, which one of the following statement is correct?
The activation energy in a chemical reaction is defined as ______.
Arrhenius equation can be represented graphically as follows:

The (i) intercept and (ii) slope of the graph are:
What happens to the rate constant k and activation energy Ea as the temperature of a chemical reaction is increased? Justify.
It is generally observed that the rate of a chemical reaction becomes double with every 10oC rise in temperature. If the generalisation holds true for a reaction in the temperature range of 298K to 308K, what would be the value of activation energy (Ea) for the reaction?
