Advertisements
Advertisements
Question
Why in the redox titration of \[\ce{KMnO4}\] vs oxalic acid, we heat oxalic acid solution before starting the titration?
Solution
The reaction between \[\ce{KMnO4}\] and oxalic acid is very slow. By raising the temperature we can increase the rate of reaction.
APPEARS IN
RELATED QUESTIONS
Consider the reaction
`3I_((aq))^-) +S_2O_8^(2-)->I_(3(aq))^-) + 2S_2O_4^(2-)`
At particular time t, `(d[SO_4^(2-)])/dt=2.2xx10^(-2)"M/s"`
What are the values of the following at the same time?
a. `-(d[I^-])/dt`
b. `-(d[S_2O_8^(2-)])/dt`
c. `-(d[I_3^-])/dt`
The rate constant of a first order reaction increases from 2 × 10−2 to 4 × 10−2 when the temperature changes from 300 K to 310 K. Calculate the energy of activation (Ea).
(log 2 = 0.301, log 3 = 0.4771, log 4 = 0.6021)
A first-order reaction is 50% completed in 40 minutes at 300 K and in 20 minutes at 320 K. Calculate the activation energy of the reaction. (Given : log 2 = 0·3010, log 4 = 0·6021, R = 8·314 JK–1 mol–1)
Activation energy of a chemical reaction can be determined by ______.
Which of the following graphs represents exothermic reaction?
(a)
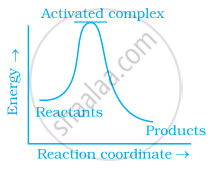
(b)
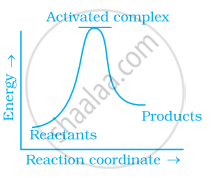
(c)
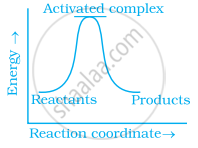
During decomposition of an activated complex:
(i) energy is always released
(ii) energy is always absorbed
(iii) energy does not change
(iv) reactants may be formed
Which of the following statements are in accordance with the Arrhenius equation?
(i) Rate of a reaction increases with increase in temperature.
(ii) Rate of a reaction increases with decrease in activation energy.
(iii) Rate constant decreases exponentially with increase in temperature.
(iv) Rate of reaction decreases with decrease in activation energy.
Mark the incorrect statements:
(i) Catalyst provides an alternative pathway to reaction mechanism.
(ii) Catalyst raises the activation energy.
(iii) Catalyst lowers the activation energy.
(iv) Catalyst alters enthalpy change of the reaction.
An exothermic reaction X → Y has an activation energy 30 kJ mol-1. If energy change ΔE during the reaction is - 20 kJ, then the activation energy for the reverse reaction in kJ is ______.
A first-order reaction is 50% complete in 30 minutes at 300 K and in 10 minutes at 320 K. Calculate activation energy (Ea) for the reaction. [R = 8.314 J K−1 mol−1]
[Given: log 2 = 0.3010, log 3 = 0.4771, log 4 = 0.6021]
