Advertisements
Advertisements
Question
Thermodynamic feasibility of the reaction alone cannot decide the rate of the reaction. Explain with the help of one example.
Solution
Thermodynamics feasibility of a reaction depends on Gibbs free energy i.e., AG must be negative for spontaneous process. Kinetic feasibility depends on the activation energy of reaction, the lesser is the activation energy, the greater is the feasibility of reaction, i.e.,
i.e., Diamond `->` Graphic ΔG = – ve
This process is thermodynamically feasible but it is very slow due to its high activation energy.
APPEARS IN
RELATED QUESTIONS
The rate constant of a first order reaction are 0.58 S-1 at 313 K and 0.045 S-1 at 293 K. What is the energy of activation for the reaction?
Calculate activation energy for a reaction of which rate constant becomes four times when temperature changes from 30 °C to 50 °C. (Given R = 8.314 JK−1 mol−1).
Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction. (Given : log2 = 0.3010,log 3 = 0.4771, log5 = 0.6990).
The rate of chemical reaction becomes double for every 10° rise in temperature because of ____________.
Which of the following graphs represents exothermic reaction?
(a)
(b)
(c)
During decomposition of an activated complex:
(i) energy is always released
(ii) energy is always absorbed
(iii) energy does not change
(iv) reactants may be formed
The reaction between \[\ce{H2(g)}\] and \[\ce{O2(g)}\] is highly feasible yet allowing the gases to stand at room temperature in the same vessel does not lead to the formation of water. Explain.
Why in the redox titration of \[\ce{KMnO4}\] vs oxalic acid, we heat oxalic acid solution before starting the titration?
An exothermic reaction X → Y has an activation energy 30 kJ mol-1. If energy change ΔE during the reaction is - 20 kJ, then the activation energy for the reverse reaction in kJ is ______.
A schematic plot of ln Keq versus inverse of temperature for a reaction is shown below
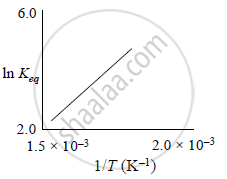
The reaction must be:
