Advertisements
Advertisements
Question
Why does the rate of any reaction generally decreases during the course of the reaction?
Solution
The rate of a reaction depends on the concentration of reactants. As the reaction progresses, the concentration of reactants decreases because the reactants start getting converted to products. Hence the rate decreases.
APPEARS IN
RELATED QUESTIONS
From the rate expression for the following reaction, determine the order of reaction and the dimension of the rate constant.
\[\ce{C2H5Cl_{(g)} -> C2H4_{(g)} + HCl_{(g)}}\] Rate = k [C2H5Cl]
A reaction is first order in A and second order in B. How is the rate affected on increasing the concentration of B three times?
What is the order of a reaction which has a rate expression; Rate = `"k"["A"]^(3/2)["B"]^1`?
Compounds ‘A’ and ‘B’ react according to the following chemical equation.
\[\ce{A(g) + 2B(g) -> 2C(g)}\]
Concentration of either ‘A’ or ‘B’ were changed keeping the concentrations of one of the reactants constant and rates were measured as a function of initial concentration. Following results were obtained. Choose the correct option for the rate equations for this reaction.
| Experiment | Initial concentration of [A]/mol L–¹ |
Initial concentration of [B]/mol L–¹ |
Initial rate of formation of [C]/mol L–¹ s–¹ |
| 1. | 0.30 | 0.30 | 0.10 |
| 2. | 0.30 | 0.60 | 0.40 |
| 3. | 0.60 | 0.30 | 0.20 |
Why molecularity is applicable only for elementary reactions and order is applicable for elementary as well as complex reactions?
Match the graph given in Column I with the order of reaction given in Column II. More than one item in Column I may link to the same item of Column II.
| Column I | Column II | |
| (i) | 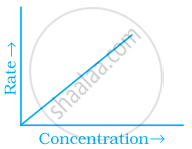 |
|
| (ii) | 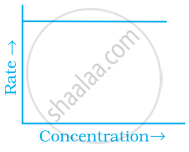 |
(a) 1st order |
| (iii) | 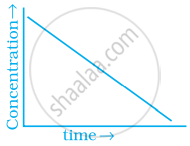 |
(b) Zero-order |
| (iv) | 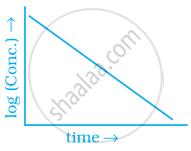 |
The role of a catalyst is to change
In the presence of a catalyst, the heat evolved or absorbed during the reaction.
If the 0.05 molar solution of m+ is replaced by a 0.0025 molar m+ solution, then the magnitude of the cell potential would be
Identify the order of reaction from the following unit for its rate constant:
L mol–1s–1
