Advertisements
Advertisements
Question
A first-order reaction is 50% completed in 40 minutes at 300 K and in 20 minutes at 320 K. Calculate the activation energy of the reaction. (Given : log 2 = 0·3010, log 4 = 0·6021, R = 8·314 JK–1 mol–1)
Solution
Given
t1/2 = 40 min at temperature (T1) = 300 K
t1/2 = 20 min at temperature (T2) = 320 K
t1/2 = 40 min, t1/2 = 20 min
`k_1 = 0.693/40`
`k_2 = 0.693/20`
According to Arrhenius equation
`log (k_2/k_1) = "E"_"a"/(2.303 " R") [1/"T"_1 - 1/"T"_2]`
`= "E"_"a"/(2.303 " R") [("T"_2 - "T"_1)/("T"_1"T"_2)]`
`log ((0.0693/20)/(0.0693/40)) = "E"_"a"/(2.303 xx 8.314) [(320 - 300)/(300 xx 320)]`
`therefore 0.3010 = "E"_"a"/19.147 [0.0002083]`
Ea = 27664 J/mol
Ea = 27.7 kJ/mol
APPEARS IN
RELATED QUESTIONS
The rate of the chemical reaction doubles for an increase of 10 K in absolute temperature from 298 K. Calculate Ea.
Consider a certain reaction \[\ce{A -> Products}\] with k = 2.0 × 10−2 s−1. Calculate the concentration of A remaining after 100 s if the initial concentration of A is 1.0 mol L−1.
The decomposition of hydrocarbon follows the equation k = `(4.5 xx 10^11 "s"^-1) "e"^(-28000 "K"//"T")`
Calculate Ea.
The decomposition of A into product has value of k as 4.5 × 103 s−1 at 10°C and energy of activation 60 kJ mol−1. At what temperature would k be 1.5 × 104 s−1?
In the Arrhenius equation for a first order reaction, the values of ‘A’ of ‘Ea’ are 4 x 1013 sec-1 and 98.6 kJ mol-1 respectively. At what temperature will its half life period be 10 minutes?
[R = 8.314 J K-1 mol-1]
Define activation energy.
What is the effect of adding a catalyst on Activation energy (Ea)
Predict the main product of the following reactions: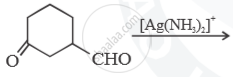
The chemical reaction in which reactants require high amount of activation energy are generally ____________.
Consider figure and mark the correct option.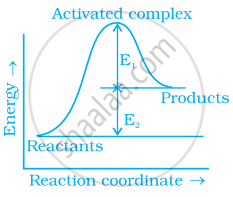
During decomposition of an activated complex:
(i) energy is always released
(ii) energy is always absorbed
(iii) energy does not change
(iv) reactants may be formed
Mark the incorrect statements:
(i) Catalyst provides an alternative pathway to reaction mechanism.
(ii) Catalyst raises the activation energy.
(iii) Catalyst lowers the activation energy.
(iv) Catalyst alters enthalpy change of the reaction.
Why does the rate of a reaction increase with rise in temperature?
Oxygen is available in plenty in air yet fuels do not burn by themselves at room temperature. Explain.
Why in the redox titration of \[\ce{KMnO4}\] vs oxalic acid, we heat oxalic acid solution before starting the titration?
Match the statements given in Column I and Column II
| Column I | Column I | |
| (i) | Catalyst alters the rate of reaction | (a) cannot be fraction or zero |
| (ii) | Molecularity | (b) proper orientation is not there always |
| (iii) | Second half life of first order reaction | (c) by lowering the activation energy |
| (iv) | `e^((-E_a)/(RT)` | (d) is same as the first |
| (v) | Energetically favourable reactions (e) total probability is one are sometimes slow | (e) total probability is one |
| (vi) | Area under the Maxwell Boltzman curve is constant | (f) refers to the fraction of molecules with energy equal to or greater than activation energy |
The rate constant for a reaction is 1.5 × 10–7 sec–1 at 50°C. What is the value of activation energy?
A schematic plot of ln Keq versus inverse of temperature for a reaction is shown below
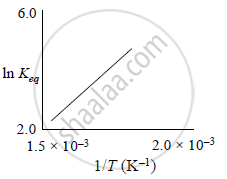
The reaction must be:
