Advertisements
Advertisements
Question
Why does the rate of a reaction increase with rise in temperature?
Solution
At higher temperatures, larger fraction of colliding particles can cross the energy barrier (i.e. the activation energy), which leads to faster rate.
APPEARS IN
RELATED QUESTIONS
The rate constant for the decomposition of hydrocarbons is 2.418 × 10−5 s−1 at 546 K. If the energy of activation is 179.9 kJ/mol, what will be the value of pre-exponential factor?
The decomposition of A into product has value of k as 4.5 × 103 s−1 at 10°C and energy of activation 60 kJ mol−1. At what temperature would k be 1.5 × 104 s−1?
The rate of a reaction quadruples when the temperature changes from 293 K to 313 K. Calculate the energy of activation of the reaction assuming that it does not change with temperature.
Calculate activation energy for a reaction of which rate constant becomes four times when temperature changes from 30 °C to 50 °C. (Given R = 8.314 JK−1 mol−1).
The rate of chemical reaction becomes double for every 10° rise in temperature because of ____________.
Activation energy of a chemical reaction can be determined by ______.
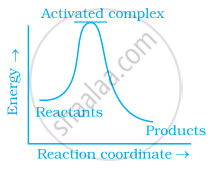
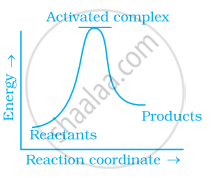
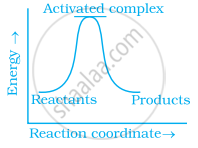
Which of the following graphs represents exothermic reaction?
(a)
(b)
(c)
Which of the following statements are in accordance with the Arrhenius equation?
(i) Rate of a reaction increases with increase in temperature.
(ii) Rate of a reaction increases with decrease in activation energy.
(iii) Rate constant decreases exponentially with increase in temperature.
(iv) Rate of reaction decreases with decrease in activation energy.
In respect of the eqn k = \[\ce{Ae^{{-E_a}/{RT}}}\] in chemical kinetics, which one of the following statement is correct?
The activation energy of one of the reactions in a biochemical process is 532611 J mol–1. When the temperature falls from 310 K to 300 K, the change in rate constant observed is k300 = x × 10–3 k310. The value of x is ______.
[Given: ln 10 = 2.3, R = 8.3 J K–1 mol–1]
