Advertisements
Advertisements
Question
Oxygen is available in plenty in air yet fuels do not burn by themselves at room temperature. Explain.
Solution
The activation energy for combustion reactions of fuels is very high at room temperature therefore they do not burn by themselves.
APPEARS IN
RELATED QUESTIONS
The rate of the chemical reaction doubles for an increase of 10 K in absolute temperature from 298 K. Calculate Ea.
The rate of a reaction quadruples when the temperature changes from 293 K to 313 K. Calculate the energy of activation of the reaction assuming that it does not change with temperature.
What is the effect of adding a catalyst on Activation energy (Ea)
Activation energy of a chemical reaction can be determined by ______.
Consider figure and mark the correct option.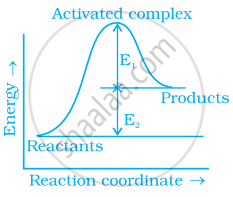
Thermodynamic feasibility of the reaction alone cannot decide the rate of the reaction. Explain with the help of one example.
In respect of the eqn k = \[\ce{Ae^{{-E_a}/{RT}}}\] in chemical kinetics, which one of the following statement is correct?
Arrhenius equation can be represented graphically as follows:

The (i) intercept and (ii) slope of the graph are:
An exothermic reaction X → Y has an activation energy 30 kJ mol-1. If energy change ΔE during the reaction is - 20 kJ, then the activation energy for the reverse reaction in kJ is ______.
What happens to the rate constant k and activation energy Ea as the temperature of a chemical reaction is increased? Justify.
