Advertisements
Advertisements
Question
Consider figure and mark the correct option.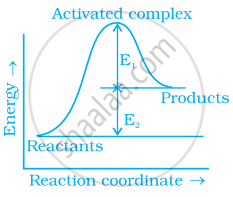
Options
Activation energy of forward reaction is E1 + E2 and product is less stable than reactant.
Activation energy of forward reaction is E1 + E2 and product is more stable than reactant.
Activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than product.
Activation energy of backward reaction is E1 and product is more stable than reactant.
Solution
Activation energy of forward reaction is E1 + E2 and product is less stable than reactant.
Explanation:
Ea (forward) = E1 + E2
Since energy of reactants is less than products and the product is less stable than the reactant.
APPEARS IN
RELATED QUESTIONS
The rate constant of a first order reaction increases from 4 × 10−2 to 8 × 10−2 when the temperature changes from 27°C to 37°C. Calculate the energy of activation (Ea). (log 2 = 0.301, log 3 = 0.4771, log 4 = 0.6021)
What will be the effect of temperature on rate constant?
The rate constant for the decomposition of hydrocarbons is 2.418 × 10−5 s−1 at 546 K. If the energy of activation is 179.9 kJ/mol, what will be the value of pre-exponential factor?
The decomposition of hydrocarbon follows the equation k = `(4.5 xx 10^11 "s"^-1) "e"^(-28000 "K"//"T")`
Calculate Ea.
Explain the following terms :
Half life period of a reaction (t1/2)
Predict the main product of the following reactions: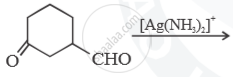
Activation energy of a chemical reaction can be determined by ______.
Match the statements given in Column I and Column II
| Column I | Column I | |
| (i) | Catalyst alters the rate of reaction | (a) cannot be fraction or zero |
| (ii) | Molecularity | (b) proper orientation is not there always |
| (iii) | Second half life of first order reaction | (c) by lowering the activation energy |
| (iv) | `e^((-E_a)/(RT)` | (d) is same as the first |
| (v) | Energetically favourable reactions (e) total probability is one are sometimes slow | (e) total probability is one |
| (vi) | Area under the Maxwell Boltzman curve is constant | (f) refers to the fraction of molecules with energy equal to or greater than activation energy |
The equation k = `(6.5 xx 10^12 "s"^(-1))"e"^(- 26000 " K"//"T")` is followed for the decomposition of compound A. The activation energy for the reaction is ______ kJ mol-1. (Nearest integer) (Given: R = 8.314 JK-1 mol-1)
A first-order reaction is 50% complete in 30 minutes at 300 K and in 10 minutes at 320 K. Calculate activation energy (Ea) for the reaction. [R = 8.314 J K−1 mol−1]
[Given: log 2 = 0.3010, log 3 = 0.4771, log 4 = 0.6021]
