Advertisements
Advertisements
Question
Predict the main product of the following reactions: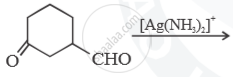
Solution
Predict main product of following reaction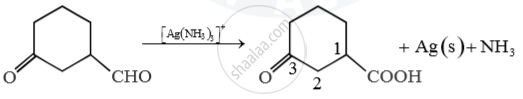
Main product 3-oxocyclohexanecarboxylic acid
APPEARS IN
RELATED QUESTIONS
What will be the effect of temperature on rate constant?
The rate of the chemical reaction doubles for an increase of 10 K in absolute temperature from 298 K. Calculate Ea.
Consider a certain reaction \[\ce{A -> Products}\] with k = 2.0 × 10−2 s−1. Calculate the concentration of A remaining after 100 s if the initial concentration of A is 1.0 mol L−1.
Which of the following graphs represents exothermic reaction?
(a)
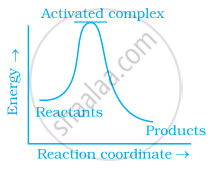
(b)
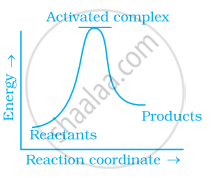
(c)
During decomposition of an activated complex:
(i) energy is always released
(ii) energy is always absorbed
(iii) energy does not change
(iv) reactants may be formed
Why does the rate of a reaction increase with rise in temperature?
Match the statements given in Column I and Column II
| Column I | Column I | |
| (i) | Catalyst alters the rate of reaction | (a) cannot be fraction or zero |
| (ii) | Molecularity | (b) proper orientation is not there always |
| (iii) | Second half life of first order reaction | (c) by lowering the activation energy |
| (iv) | `e^((-E_a)/(RT)` | (d) is same as the first |
| (v) | Energetically favourable reactions (e) total probability is one are sometimes slow | (e) total probability is one |
| (vi) | Area under the Maxwell Boltzman curve is constant | (f) refers to the fraction of molecules with energy equal to or greater than activation energy |
In respect of the eqn k = \[\ce{Ae^{{-E_a}/{RT}}}\] in chemical kinetics, which one of the following statement is correct?
The activation energy in a chemical reaction is defined as ______.
The activation energy in a chemical reaction is defined as ______.
