Science (English Medium)
Academic Year: 2018-2019
Date & Time: 13th March 2019, 10:30 am
Duration: 3h
Advertisements
Write IUPAC name of the complex K3 [Cr (C2O4)3]
Chapter: [0.05] Coordination Compounds
Using IUPAC norms write the formula of Hexaamminecobalt (III) sulphate.
Chapter: [0.09] Amines
Why is CH2 = CH - CH2 - Cl more easily hydrolysed than CH3 - CH2 - CH2 - Cl ?
Chapter: [0.06] Haloalkanes and Haloarenes
Write the IUPAC name of the follow
\[\begin{array}\ce{\phantom{--..}O}\\\ce{\phantom{--..}||}\\ \ce{CH2=CH-C-CH3}\end{array}\]
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
What is the difference between a glycosidic linkage and a peptide linkage?
Chapter: [0.1] Biomolecules
What is the difference between a glycosidic linkage and a peptide linkage?
Chapter: [0.1] Biomolecules
Arrange the following increasing order of their boiling point:
CH3CH2OH, CH3CHO, CH3–O–CH3
Chapter: [0.01] Solutions
What happens when AgCl is doped with 2? CdCl What is the name of this defect?
Chapter: [0.01] Solid State
What type of defect is shown by NaCl in
stoichiometric defects, and
Chapter: [0.01] Solid State
Account for the following:
Gabriel phthalimide synthesis is not preferred for preparing aromatic primary amines.
Chapter: [0.09] Amines
Account for the following:
On reaction with benzene sulphonyl chloride, primary amine yields product soluble in alkali whereas secondary amine yields product insoluble in alkali.
Chapter: [0.09] Amines
Complete and balance the following equations:
S+H2SO4(conc.) →
Chapter: [0.07] P - Block Elements
Complete and balance the following equations:
PCL3 + H2O →
Chapter: [0.07] P - Block Elements
Write balanced chemical equations involved in the following reactions:
Chlorine gas reacts with cold and dilute NaOH
Chapter: [0.07] P - Block Elements
Write balanced chemical equations involved in the following reactions:
Calcium phosphide is dissolved in water.
Chapter: [0.07] P - Block Elements
Draw structures of the following:
XeF4
Chapter: [0.07] P - Block Elements
Draw structures of the following:
`S_2O_8^{2-}`
Chapter: [0.07] P - Block Elements
Write the reaction that occurs at anode on electrolysis of concentrated 2 4 H SO using platinum electrodes.
Chapter: [0.02] Electrochemistry
What is the effect of temperature on ionic conductance?
Chapter: [0.02] Electrochemistry
Out of 0.1 molal aqueous solution of glucose and 0.1 molal aqueous solution of KCl, which one will have higher boiling point and why?
Chapter: [0.01] Solutions
Predict whether van’t Hoff factor, (i) is less than one or greater than one in the following:
CH3COOH dissolved in water
Chapter: [0.01] Solutions
Write structures of compounds A and B in each of the following reactions: 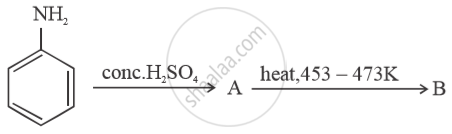
Chapter: [0.09] Amines
Write structures of compounds A and B in each of the following reactions: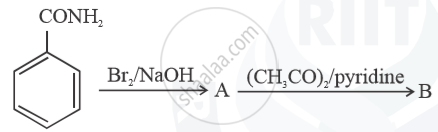
Chapter: [0.09] Amines
Define the following with a suitable example, of each:
Coagulation
Chapter: [0.05] Surface Chemistry
Define the following with a suitable example, of each:
Multimolecular colloid
Chapter: [0.05] Surface Chemistry
Define the following with a suitable example, of each:
Gel
Chapter: [0.05] Surface Chemistry
Out of starch and ferric hydroxide sol, which one can easily be coagulated and why?
Chapter: [0.05] Surface Chemistry
What is observed when an emulsion is centrifuged?
Chapter: [0.05] Surface Chemistry
What is the role of promoters and poisons in catalysis?
Chapter: [0.05] Surface Chemistry
Advertisements
Following reaction takes place in the cell:
`Zn(s) + Ag_2O(s)+H_2O(l) -> Zn^{2+}(aq) + 2Ag (s) + 2OH^- (aq)`
Calculate `Delta_r G^0` of the reaction
[Given ; `E^0_(Zn^{2+}//Zn)` = -0.76V
`E_((Zn^{2+}//Zn)) = 0.76V`
`E_(Ag^4//Ag)^0 = 0.80V, 1F = 96,500 C mol^-1 ]`
Chapter: [0.02] Electrochemistry
How can you determine limiting molar conductivity, 0 m for strong electrolyte and weak electrolyte?
Chapter: [0.02] Electrochemistry
Write down the reaction taking place in blast furnace related to the metallurgy of iron in the temperature rnge 500 K – 800 K. What is the role of limestone in the metallurgy of iron?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What happens when Silver is leached with NaCN in the presence of air?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What happens when Copper matte is charged into silica lined converter and hot air blast is blown?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What happens when Silver is leached with NaCN in the presence of air?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
A solution 0.1 M of Na2SO4 is dissolved to the extent of 95%. What would be its osmotic pressure at 027 ? C (R = 0.0821 L atm K-1 mol-1) .
Chapter: [0.01] Solutions
An element crystallises in bcc lattic with a cell edge of 3 × 10-8cm. The density of the element is 6.89 g cm-3 . calculate the molar mass of element. (NA = 6.022 × 1023 mol-1)
Chapter: [0.01] Solid State
What type of semiconductor is obtained when
Ge is doped with In?
Chapter: [0.01] Solid State
What type of semiconductor is obtained when
Si is doped with P?
Chapter: [0.01] Solid State
Give reactions for the following:
O – O single bond is weaker than S – S single bond.
Chapter: [0.07] P - Block Elements
Tendency to show –3 oxidation state decrease from Nitrogn (N) to Bismuth (Bi).
Chapter: [0.07] P - Block Elements
Cl2 acts as a bleaching agent.
Chapter: [0.07] P - Block Elements
Write the structures of monomers used the following polymers:
Buna S
Chapter: [0.15] Polymers
Write the structures of monomers used the following polymers:
Glyptal
Chapter: [0.15] Polymers
Write the structures of monomers used to obtain the following polymers:
Nylon-6
Chapter:
Arrange the following polymers in increasing order of their intermolecular forces:
Polyvinylchloride, Neoprene, Terylene
Chapter: [0.15] Polymers
Write one example each of :
Natural polymer
Chapter: [0.1] Biomolecules
Write one example each of :
Thermosetting polymer
Chapter: [0.1] Biomolecules
What is the significance of number of 6,6 in the polymer nylon-6,6?
Chapter: [0.15] Polymers
Which one of the following is a disinfectant?
0.2% solution of phenol or 1% solution of phenol
Chapter: [0.16] Chemistry in Everyday Life
What is the difference between agonists and antagonists?
Chapter: [0.16] Chemistry in Everyday Life
Write one example each of Artificial sweetener.
Chapter: [0.16] Chemistry in Everyday Life
Write one example each of Antacids.
Chapter: [0.16] Chemistry in Everyday Life
Define the following terms with a suitable example of each:
Antiseptics
Chapter: [0.16] Chemistry in Everyday Life
Define the following terms with a suitable example of each:
Bactericidal antibiotics
Chapter: [0.16] Chemistry in Everyday Life
Define the following term with a suitable example:
Cationic detergents
Chapter: [0.16] Chemistry in Everyday Life
What happens when Salicylic acid is treated with (CH3CO)2 O/H+?
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
What happens when Phenol is oxidised with Na2Cr2O7/H+?
Chapter: [0.07] Alcohols, Phenols and Ethers
What happens when Anisole is treated with CH3Cl/anhydrous AlCl3?
Chapter: [0.07] Alcohols, Phenols and Ethers
Advertisements
Write chemical equation in support of your answer.
Out of 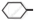 Cl and
Cl and 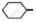 CH2- Cl, which one is more reactive towards nucleophilic substitution reaction and why?
CH2- Cl, which one is more reactive towards nucleophilic substitution reaction and why?
Chapter: [0.06] Haloalkanes and Haloarenes
Write chemical equation in support of your answer.
Out of 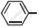 Cl and O2N
Cl and O2N  Cl. which one is more reactive towards nucleophilic substitution reaction and why?
Cl. which one is more reactive towards nucleophilic substitution reaction and why?
Chapter: [0.09] Amines
Out of 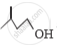 and
and 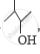 , which one is optically active and why ?
, which one is optically active and why ?
Chapter: [0.05] Coordination Compounds
Give one chemical test as an evidence to show that [Co (NH3)5Cl] are ionisation isomers.
Chapter: [0.05] Coordination Compounds
[NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why? (Atomic no. Ni = 28)
Chapter: [0.05] Coordination Compounds
Write the electronic configuration of Fe(III) on the basis of crystal field theory when it forms an octahedral complex in the presence of (i) strong field, and (ii) weak field ligand. (Atomic no.of Fe=26)
Chapter: [0.05] Coordination Compounds
What is the difference between fibrous protein and globular protein ?
Chapter: [0.1] Biomolecules
Differentiable between the following:
Essential amino acids Non-essential amino acids
Chapter: [0.1] Biomolecules
Differentiable between the following:
Amylose and Amylopectin
Chapter: [0.1] Biomolecules
Account for the following :
Manganese shows maximum number of oxidation states in 3d series.
Chapter: [0.04] d-block and f-block Elements
E0 value for Mn3+ Mn2+ couple is much more positive than that for Cr3-/ Cr2-.
Chapter: [0.04] d-block and f-block Elements
Account for the following :
Ti4+ is colourless whereas V4+ is coloured in an aqueous solutions.
Chapter: [0.04] d-block and f-block Elements
Write the chemical equation for the preparation of 4 KMnO from 2. MnO Why does purple colour of acidified permanganate solution decolourise when it oxidises Fe2- to Fe3+ ?
Chapter: [0.04] d-block and f-block Elements
Write one difference between transition elements and p-block elements with reference to variability of oxidation states.
Chapter: [0.07] P - Block Elements
Why do transition metals exhibit higher enthalpy of atomization?
Chapter: [0.04] d-block and f-block Elements
Name an element of lanthanoid series which is well knwon to shown +4 oxidation state. Is it a strong oxidising agent or reducing agent?
Chapter: [0.04] d-block and f-block Elements
What is lanthanoid contraction? Write the.............
Chapter: [0.04] d-block and f-block Elements
Write the ionic equation showing the oxidation of Fe(II) salt by acidified dichromate solutions.
Chapter: [0.04] d-block and f-block Elements
Define order of reaction. How does order of a reaction differ from molecularity for a complex reaction?
Chapter: [0.03] Chemical Kinetics
A first order reaction is 50% complete in 25 minutes. Calculate the time for 80% completion of the reaction.
Chapter: [0.03] Chemical Kinetics
The decomposition of a hydrocarbon has value of rate constant as 2.5×104s-1 At 27° what temperature would rate constant be 7.5×104 × 3 s-1if energy of activation is 19.147 × 103 J mol-1 ?
Chapter: [0.03] Chemical Kinetics
Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction. (Given : log2 = 0.3010,log 3 = 0.4771, log5 = 0.6990).
Chapter: [0.03] Chemical Kinetics
Predict the main product of the following reactions: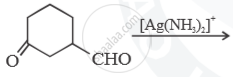
Chapter: [0.03] Chemical Kinetics
Predict the main product of the following reaction:\[\begin{array}{c}
\ce{O\phantom{----}O\phantom{-}}\\
\ce{||\phantom{----}||\phantom{-}}\\
\ce{CH3-C-CH2-C-OCH3}
\end{array}\ce{->[(i)NaBH4][(ii)H+]}\]
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Predict the main product of the following reactions: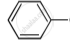 \[\ce{CHO + CH_3CHO->[dil NaOH]}\]
\[\ce{CHO + CH_3CHO->[dil NaOH]}\]
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Give a simple chemical test to distinguish between 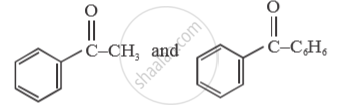
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Why is alpha (α) hydrogen of carbonyl compounds acidic in nature?
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the main product formed when propanal reacts with the following reagents:
2 moles of 3 CH OH in presence of dry HCl
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the main product formed when propanal reacts with the following reagents:
(ii) Dilute NaOH
`CH_3CH_2CHO + NaOH(dil)-> `
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the main product formed when propanal reacts with the following reagents:
H2N- NH2 followed by heating with KOH in ethylene glycol.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Arrange the following compounds in increasing order of their property as indicated:
F - CH2COOH, O2N - CH2 COOH CH3 COOH,HCOOH - acid character.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Acetone, Acetaldehyde, Benzaldehyde, Acetophenone – reactivity towards addition of HCN.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 12 Chemistry with solutions 2018 - 2019
Previous year Question paper for CBSE Class 12 -2019 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 12.
How CBSE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
