Advertisements
Advertisements
Question
E0 value for Mn3+ Mn2+ couple is much more positive than that for Cr3-/ Cr2-.
Solution
Much larger 3rd ionisation energy of Mn (where the required change is d5 to d4) is mainly responsible for this.
Mn3+ = 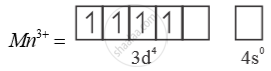
Mn2+ = 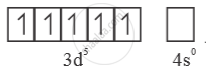 → Half filled (more stable)
→ Half filled (more stable)
APPEARS IN
RELATED QUESTIONS
Complete the following equation :
`2MnO_4^(-)+6H^++5NO_2^(-)rarr`
Complete the following equations : 2 MnO2 + 4 KOH + O2 →
Complete the following equation :
Complete the following chemical equations :
Cu + H2SO4(conc.) →
Account for the following :
Manganese shows maximum number of oxidation states in 3d series.
Which of the following reactions are disproportionation reactions?
(a) \[\ce{Cu^{+} -> Cu^{2+} + Cu}\]
(b) \[\ce{3MnO^{-}4 + 4H^{+} -> 2MnO^{-}4 + MnO2 + 2H2O}\]
(c) \[\ce{2KMnO4 -> K2MnO4 + MnO2 + O2}\]
(d) \[\ce{2MnO^{-}4 + 3Mn^{2+} + 2H2O -> 5MnO2 + 4H^{+}}\]
KMnO4 is coloured due to ______.
What is the effect of pH on dichromate ion solution?
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
