Advertisements
Advertisements
Question
Account for the following :
Manganese shows maximum number of oxidation states in 3d series.
Solution
Mnexhibits all the oxidation states from (+ 2) to (+ 7)
`"_25Mn = [Ar]3d^s 4s^2`
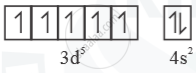
Mn+ = 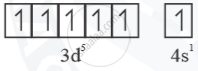 Half filled (stable)
Half filled (stable)
Mn2+ = 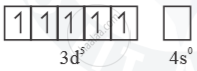
Mn3 = 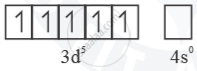
Mn4+= 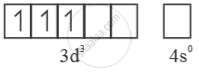
Mn5+ = 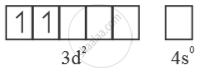
Mn6+ = 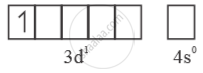
Mn+7 = 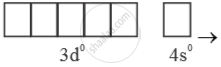 → Highest oxidation state = (+7)
→ Highest oxidation state = (+7)
APPEARS IN
RELATED QUESTIONS
Complete the following equation :
`2MnO_4^(-)+6H^++5NO_2^(-)rarr`
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with iron (II) ions? Write the ionic equation for the reaction.
Indicate the steps in the preparation of KMnO4 from pyrolusite ore.
E0 value for Mn3+ Mn2+ couple is much more positive than that for Cr3-/ Cr2-.
Zinc carbonate is precipitated from zinc sulphate solution by the addition of ___________.
When \[\ce{Cu^2+}\] ion is treated with \[\ce{KI}\], a white precipitate is formed. Explain the reaction with the help of chemical equation.
Complete the following ionic equation:
\[\ce{Cr2O^{2-}7 + 2OH^- ->}\]
Indicate the step in the preparation of K2Cr2O7 from chromite ore.
Indicate the steps in the preparation of K2Cr2O7 from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
