Advertisements
Advertisements
प्रश्न
Account for the following :
Manganese shows maximum number of oxidation states in 3d series.
उत्तर
Mnexhibits all the oxidation states from (+ 2) to (+ 7)
`"_25Mn = [Ar]3d^s 4s^2`
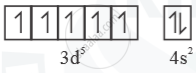
Mn+ = 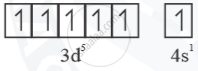 Half filled (stable)
Half filled (stable)
Mn2+ = 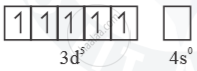
Mn3 = 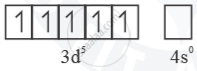
Mn4+= 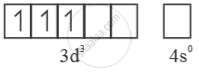
Mn5+ = 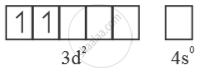
Mn6+ = 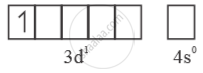
Mn+7 = 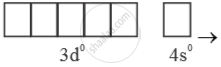 → Highest oxidation state = (+7)
→ Highest oxidation state = (+7)
APPEARS IN
संबंधित प्रश्न
Complete the following equation :
`2MnO_4^(-)+6H^++5NO_2^(-)rarr`
Complete the following equations : 2 MnO2 + 4 KOH + O2 →
Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with iron (II) ions? Write the ionic equation for the reaction.
Indicate the steps in the preparation of KMnO4 from pyrolusite ore.
The oxidation state of manganese in the product obtained in a reaction of potassium permanganate and hydrogen peroxide in a basic medium is ______.
Complete the reaction mentioning all the products formed:
\[\ce{Cr2O^{2-}7 + 3H2S + 8H^+ ->}\]
Indicate the steps in the preparation of \[\ce{K2Cr2O2}\] from chromite ore.
Indicate the step in the preparation of K2Cr2O7 from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
