Advertisements
Advertisements
प्रश्न
Indicate the steps in the preparation of KMnO4 from pyrolusite ore.
उत्तर १
Potassium permanganate can be made by the following methods –
It is produced industrially by the electrolytic oxidation of manganate(VI) followed by the alkaline oxidative fusion of MnO2.
\[\ce{MnO2 ->[{Fused with KOH, oxidised with air or KNO3}] \underset{{manganate ion}}{MnO^{2-}_4}}\]
\[\ce{\underset{{manganate ion}}{MnO^{2-}_4} ->[{Electrolytic oxidation in alkaline solution}] \underset{{permanganate ion}}{MnO^-_4}}\]
The chemical reactions of acidic potassium permanganate with SO2 are as follows –
\[\ce{2MnO4 + 2H2O + 5SO2 -> 2Mn^{2+} + 4H^+ + 5SO^{2-}_4}\]
उत्तर २
\[\ce{2MnO2 + 4KOH + O2 ->[\Delta] 2K2MnO4 + 2H2O}\]
\[\ce{3MnO^2-_4 + 4H+ -> 2MnO^-_4 + MnO2 + 2H2O}\]
APPEARS IN
संबंधित प्रश्न
Complete the following equations:
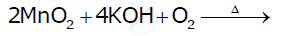
Complete the following chemical equation
8MnO4- + 3S2O32- + H2O →
Complete the following equations : 2 MnO2 + 4 KOH + O2 →
Complete the following equations : MnO4- + 4H+ + 3e- →
Complete the following equation : MnO4- + 8H+ + 5e- →
Write the formula of an oxo-anion of Manganese (Mn) in which it shows the oxidation state equal to its group number.
Complete the following equations:
`2MnO_4^(-)+16H^++5S^(2-)rarr`
Complete the following chemical equations :
Cu + H2SO4(conc.) →
Write the chemical equation for the preparation of 4 KMnO from 2. MnO Why does purple colour of acidified permanganate solution decolourise when it oxidises Fe2- to Fe3+ ?
Answer the following question.
When MnO2 is fused with KOH in the presence of KNO3 as an oxidizing agent, it gives a dark green compound (A). Compound (A) disproportionates in an acidic solution to give a purple compound (B). An alkaline solution of compound (B) oxidizes KI to compound (C) whereas an acidified solution of compound (B) oxidizes KI to (D). Identify (A), (B), (C), and (D).
On addition of small amount of \[\ce{KMnO4}\] to concentrated \[\ce{H2SO4}\], a green oily compound is obtained which is highly explosive in nature. Identify the compound from the following.
Which of the following reactions are disproportionation reactions?
(a) \[\ce{Cu^{+} -> Cu^{2+} + Cu}\]
(b) \[\ce{3MnO^{-}4 + 4H^{+} -> 2MnO^{-}4 + MnO2 + 2H2O}\]
(c) \[\ce{2KMnO4 -> K2MnO4 + MnO2 + O2}\]
(d) \[\ce{2MnO^{-}4 + 3Mn^{2+} + 2H2O -> 5MnO2 + 4H^{+}}\]
Generally transition elements and their salts are coloured due to the presence of unpaired electrons in metal ions. Which of the following compounds are coloured?
(i) \[\ce{KMnO4}\]
(ii) \[\ce{Ce(SO4)2}\]
(iii) \[\ce{TiCl}\]
(iv) \[\ce{Cu2 Cl2}\]
Potassium permanganate acts as an oxidant in neutral, alkaline as well as acidic media. The final products obtained from it in the three conditions are, respectively.
Complete the following ionic equation:
\[\ce{Cr2O^{2-}7 + 2OH^- ->}\]
Which of the following ions will have a magnetic moment value of 1.73 BM.
\[\ce{Sc^3+, Ti^3+, Ti^2+, Cu^2+, Zn^2+}\]
Indicate the steps in the preparation of K2Cr2O7 from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with oxalic acid? Write the ionic equation for the reaction.
