Advertisements
Advertisements
प्रश्न
Complete the following equations : MnO4- + 4H+ + 3e- →
उत्तर
MnO4- + 4H+ + 3e- → MnO2 + 2H2O
APPEARS IN
संबंधित प्रश्न
Complete the following equations:
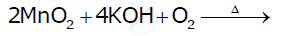
Complete the following equations : 2 MnO2 + 4 KOH + O2 →
Write the chemical equation for the preparation of 4 KMnO from 2. MnO Why does purple colour of acidified permanganate solution decolourise when it oxidises Fe2- to Fe3+ ?
Generally transition elements and their salts are coloured due to the presence of unpaired electrons in metal ions. Which of the following compounds are coloured?
(i) \[\ce{KMnO4}\]
(ii) \[\ce{Ce(SO4)2}\]
(iii) \[\ce{TiCl}\]
(iv) \[\ce{Cu2 Cl2}\]
The product formed upon heating methyl bromide with potassium tert-butoxide is
What is the effect of pH on dichromate ion solution?
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
