Advertisements
Advertisements
प्रश्न
Complete the reaction mentioning all the products formed:
\[\ce{Cr2O^{2-}7 + 3H2S + 8H^+ ->}\]
उत्तर
\[\ce{Cr2O^{2-}7 + 3H2S + 8H^+ -> 2Cr^{3+} + 7H2O + 3S}\]
Dichromate oxidises hydrogen sulphide to sulphur in an acidic medium.
APPEARS IN
संबंधित प्रश्न
Complete the following equations:
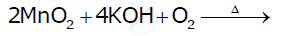
Give an example and suggest a reason for the following feature of the transition metal chemistry:
A transition metal exhibits the highest oxidation state in oxides and fluorides.
Zinc carbonate is precipitated from zinc sulphate solution by the addition of ___________.
\[\ce{KMnO4}\] acts as an oxidising agent in alkaline medium. When alkaline \[\ce{KMnO4}\] is treated with \[\ce{KI}\], iodide ion is oxidised to ______.
When \[\ce{Cu^2+}\] ion is treated with \[\ce{KI}\], a white precipitate is formed. Explain the reaction with the help of chemical equation.
When orange solution containing \[\ce{Cr2O^{2-}7}\] ion is treated with an alkali, a yellow solution is formed and when \[\ce{H^+}\] ions are added to yellow solution, an orange solution is obtained. Explain why does this happen?
Why \[\ce{HCl}\] should not be used for potassium permanganate titrations?
In the two tetrahedral structures of dichromate ion, ______.
Complete the reaction mentioning all the products formed:
\[\ce{2KMnO4 ->[\Delta]}\]
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
