Advertisements
Advertisements
प्रश्न
Account for the following :
Manganese shows maximum number of oxidation states in 3d series.
उत्तर
Mnexhibits all the oxidation states from (+ 2) to (+ 7)
`"_25Mn = [Ar]3d^s 4s^2`
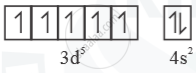
Mn+ = 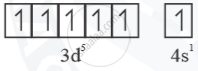 Half filled (stable)
Half filled (stable)
Mn2+ = 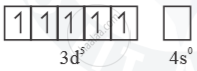
Mn3 = 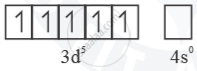
Mn4+= 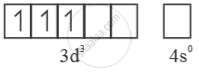
Mn5+ = 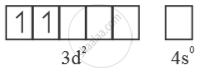
Mn6+ = 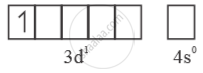
Mn+7 = 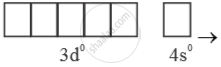 → Highest oxidation state = (+7)
→ Highest oxidation state = (+7)
APPEARS IN
संबंधित प्रश्न
Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Indicate the steps in the preparation of K2Cr2O7 from chromite ore.
Complete the following equations:
`2MnO_4^(-)+16H^++5S^(2-)rarr`
Using IUPAC norms write the formulae of Potassium trioxalatochromate (III)
Which of the following reactions are disproportionation reactions?
(a) \[\ce{Cu^{+} -> Cu^{2+} + Cu}\]
(b) \[\ce{3MnO^{-}4 + 4H^{+} -> 2MnO^{-}4 + MnO2 + 2H2O}\]
(c) \[\ce{2KMnO4 -> K2MnO4 + MnO2 + O2}\]
(d) \[\ce{2MnO^{-}4 + 3Mn^{2+} + 2H2O -> 5MnO2 + 4H^{+}}\]
\[\ce{KMnO4}\] acts as an oxidising agent in alkaline medium. When alkaline \[\ce{KMnO4}\] is treated with \[\ce{KI}\], iodide ion is oxidised to ______.
When \[\ce{Cu^2+}\] ion is treated with \[\ce{KI}\], a white precipitate is formed. Explain the reaction with the help of chemical equation.
Which of the following ions will have a magnetic moment value of 1.73 BM.
\[\ce{Sc^3+, Ti^3+, Ti^2+, Cu^2+, Zn^2+}\]
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
