Advertisements
Advertisements
प्रश्न
E0 value for Mn3+ Mn2+ couple is much more positive than that for Cr3-/ Cr2-.
उत्तर
Much larger 3rd ionisation energy of Mn (where the required change is d5 to d4) is mainly responsible for this.
Mn3+ = 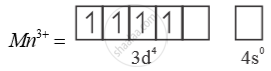
Mn2+ = 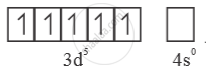 → Half filled (more stable)
→ Half filled (more stable)
APPEARS IN
संबंधित प्रश्न
When chromite ore FeCr2O4 is fused with NaOH in presence of air, a yellow-coloured compound (A) is obtained, which on acidification with dilute sulphuric acid gives a compound (B). Compound (B) on reaction with KCl forms an orange coloured crystalline compound (C).
(i) Write the formulae of the compounds (A), (B) and C.
(ii) Write one use of compound (C).
Complete the following equations : 2 MnO2 + 4 KOH + O2 →
Complete the following chemical equation
Cr2O72- + 3Sn2+ + 14H+ →
Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Give an example and suggest a reason for the following feature of the transition metal chemistry:
The lowest oxide of transition metal is basic, the highest is amphoteric/acidic.
Complete the following chemical equations :
Cu + H2SO4(conc.) →
Zinc carbonate is precipitated from zinc sulphate solution by the addition of ___________.
What is the effect of pH on dichromate ion solution?
Complete the reaction mentioning all the products formed:
\[\ce{Cr2O^{2-}7 + 3H2S + 8H^+ ->}\]
Indicate the step in the preparation of K2Cr2O7 from chromite ore.
