Advertisements
Advertisements
Question
Account for the following:
Gabriel phthalimide synthesis is not preferred for preparing aromatic primary amines.
Solution
Gabriel phthalimide synthesis not prefered for preparing aromatic primary amines because amine formation involves nucleophilic substituion 2 ( ) SN of alkyl halides by the anion formed by the phthalimide. But aryl halides do not undergo nucleophilic substitution with the anion formed by the phthalimide. As nucleophilic substitution reactions are very difficult in aryl halides.
APPEARS IN
RELATED QUESTIONS
How do you convert the following: Ethanenitrile to ethanamine
Give plausible explanation for each of the following :
Why are amines less acidic than alcohols of comparable molecular masses?
Give the structures of A, B and C in the following reactions :
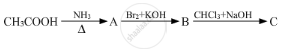
Write structures of compounds A and B in each of the following reactions: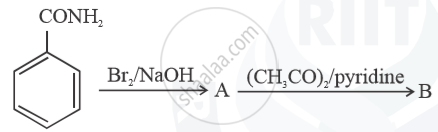
Alkyl cyanides on reduction by sodium and ethanol give primary amines. This reaction is called as ____________.
Amongst the following, the strongest base in aqueous medium is ______.
Identify the product ‘C’ in the following reaction.
\[\ce{Aniline ->[(CH3CH)2O][Pyridine] A ->[Br2][CH3COOH] B ->[H^+ or OH^-] C}\]
Write the name of the product formed by the action of LiAlH4/ether on acetamide.
Write a short note on the following:
Ammonolysis
Write short note on the following.
Ammonolysis.
