Advertisements
Advertisements
Question
non-stoichiometric defects?
Solution
The defect shown by NaCl in non-stoichiometric defect is metal excess defect due to anionic vacancies which is known as F-centre.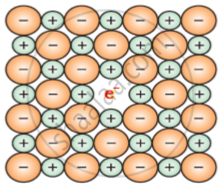
APPEARS IN
RELATED QUESTIONS
Ionic solids containing large differences in sizes of ions show ____________.
Dislocation defect is also known as ____________.
Frenkel defects are not found in alkali metal halides because ____________.
The radius of Cs+ is 169 pm and Cl− is 181 pm. The radius ratio is ____________.
Assertion: Due to Frenkel defect, there is no effect on the density of the crystalline solid.
Reason: In Frenkel defect, no cation or anion leaves the crystal.
Which of the following crystals does not exhibit Frenkel defect?
What is the effect of Frenkel defect on the density of ionic solids?
In a Schottky defect ____________.
Cations are present in the interstitial sites in ______.
Given below are two statements, one is labelled as Assertion (A) and the other is labelled as Reason (R):
Assertion: In any ionic solid (MX) with Schottky defects, the number of positive and negative ions are same.
Reason: Equal number of cation and anion vacancies are present.
