Advertisements
Advertisements
Question
Write the chemical equation for the preparation of 4 KMnO from 2. MnO Why does purple colour of acidified permanganate solution decolourise when it oxidises Fe2- to Fe3+ ?
Solution
Chemical equations for the preparation of KMnO4 from MnO2
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
`3MnO_4^2+4H -> 2MnO_4^- + 2 MnO_4^- + MnO_2 + 2H_2O `
\[\ce{ MnO_2 ->[Fused with oxidised][with air or KNO3]}MnO_4^{2-}\] maganate ion
\[\ce{ \underset{Manganate}{MnO_4^{2-}} ->[Electrolytic oxidation][in alkaline solution] MnO_4^- }\] permanganateion
Purple colour of acidified permanganate solution decolourise when it oxidises Fe2- + Fe3+
In acidic medium, Reduction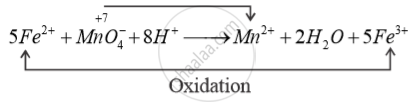
KMnO4 is behaving as oxidising Agent.
So, purple colour of acidified 4 KMnO solutions decolourises.
APPEARS IN
RELATED QUESTIONS
Complete the following equation:
\[\ce{2MnO4- + 6H+ + 5NO2- ->}\]
Complete the following equations : 2 MnO2 + 4 KOH + O2 →
Complete the following equation : MnO4- + 8H+ + 5e- →
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with iron (II) ions? Write the ionic equation for the reaction.
When \[\ce{Cu^2+}\] ion is treated with \[\ce{KI}\], a white precipitate is formed. Explain the reaction with the help of chemical equation.
The product formed upon heating methyl bromide with potassium tert-butoxide is
In the two tetrahedral structures of dichromate ion, ______.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chomite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with SO2? Write the ionic equation for the reaction.
